Volume 10, Issue 2 (6-2025)
J Res Dent Maxillofac Sci 2025, 10(2): 103-110 |
Back to browse issues page
Ethics code: IR.IAU.DENTAL.REC.1401.082
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mohitmafi N, Parsania M, Alaee A, Olyaee P, Vahedi M. In Vitro Effect of Photodynamic Therapy with 660 nm Diode Laser and Methylene Blue Photosensitizer on Acyclovir-Resistant Herpes Simplex Virus Type 1. J Res Dent Maxillofac Sci 2025; 10 (2) :103-110
URL: http://jrdms.dentaliau.ac.ir/article-1-800-en.html
URL: http://jrdms.dentaliau.ac.ir/article-1-800-en.html
1- Department of Oral and Maxillofacial Medicine, TeMS.C., Islamic Azad University, Tehran, Iran.
2- Department of Microbiology, Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran.
3- Department of Oral Medicine, Dental Material Research Center, TeMS.C., Islamic Azad University, Tehran, Iran.
4- Department of Orthodontics, Urmia University of Medical Science, School of Dentistry, Urmia, Iran.
5- Department of Oral and Maxillofacial Medicine, TeMS.C., Islamic Azad University, Tehran, Iran. ,vahedi_md@yahoo.com
2- Department of Microbiology, Faculty of Medicine, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran.
3- Department of Oral Medicine, Dental Material Research Center, TeMS.C., Islamic Azad University, Tehran, Iran.
4- Department of Orthodontics, Urmia University of Medical Science, School of Dentistry, Urmia, Iran.
5- Department of Oral and Maxillofacial Medicine, TeMS.C., Islamic Azad University, Tehran, Iran. ,
Full-Text [PDF 418 kb]
(113 Downloads)
| Abstract (HTML) (267 Views)
Full-Text: (65 Views)
Abstract
Background and Aim: Herpes simplex virus type 1 (HSV-1) is one of the most common infections of the mouth and face. This study was conducted to assess the effect of photodynamic therapy (PDT) with 660 nm diode laser and methylene blue photosensitizer (PS) with different concentrations and radiation parameters on acyclovir-resistant HSV-1.
Materials and Methods: In this in vitro study, first the cytotoxicity of methylene blue for HeLa cells was assessed by the methyl thiazolyl tetrazolium (MTT) assay, and its highest non-toxic concentrations were found (0.001%, 0.002%, 0.005%, and 0.01%). Laser was used with 660 nm wavelength, 100 mW power, and 10, 20, and 30 J/cm2 energy densities for 50, 100, and 150 seconds. The samples were divided into 20 groups, including 12 PDT groups with laser and methylene blue, 3 groups subjected to laser irradiation alone, 4 groups exposed to methylene blue alone with different concentrations, and one negative control group. After laser irradiation, HSV-1 was titrated by the tissue culture infectious dose 50 (TCID50) method. Two-way ANOVA was used for data analysis (alpha=0.05).
Results: The effects of methylene blue concentration (0.001%, 0.002%, 0.005%, and 0.01%) (P=0.675) and laser energy density (10, 20, 30 J/cm2) (P=0.914), and their interaction effect (P=0.977) on the titer of acyclovir-resistant HSV-1 were not significant.
Conclusion: PDT with 660 nm laser with different energy densities and different methylene blue concentrations had no significant effect on acyclovir-resistant HSV-1.
Keywords: Acyclovir; Photochemotherapy; Methylene Blue; Herpes Simplex virus
Introduction
Materials and Methods: In this in vitro study, first the cytotoxicity of methylene blue for HeLa cells was assessed by the methyl thiazolyl tetrazolium (MTT) assay, and its highest non-toxic concentrations were found (0.001%, 0.002%, 0.005%, and 0.01%). Laser was used with 660 nm wavelength, 100 mW power, and 10, 20, and 30 J/cm2 energy densities for 50, 100, and 150 seconds. The samples were divided into 20 groups, including 12 PDT groups with laser and methylene blue, 3 groups subjected to laser irradiation alone, 4 groups exposed to methylene blue alone with different concentrations, and one negative control group. After laser irradiation, HSV-1 was titrated by the tissue culture infectious dose 50 (TCID50) method. Two-way ANOVA was used for data analysis (alpha=0.05).
Results: The effects of methylene blue concentration (0.001%, 0.002%, 0.005%, and 0.01%) (P=0.675) and laser energy density (10, 20, 30 J/cm2) (P=0.914), and their interaction effect (P=0.977) on the titer of acyclovir-resistant HSV-1 were not significant.
Conclusion: PDT with 660 nm laser with different energy densities and different methylene blue concentrations had no significant effect on acyclovir-resistant HSV-1.
Keywords: Acyclovir; Photochemotherapy; Methylene Blue; Herpes Simplex virus
Introduction
Herpes simplex virus (HSV), which belongs to the Herpesviridae family, can cause infection throughout human life. Primary HSV infections mostly affect the mucosal surfaces, resulting in the involvement of epithelial cells and manifesting as herpes, genital herpes, herpetic herpes, encephalitis, or stromal keratitis [1]. This virus is capable of infecting various cell types, but it mainly targets the epithelial cells and nerve cells, both in the initial and relapse phases of the disease [2]. To date, two types of HSV have been identified: HSV type 1 (HSV-1) and HSV type 2 (HSV-2). HSV-1 is usually associated with oral infections, causing cold sores or fever blisters [3]. Since these infections are subclinical, most infected individuals are not aware of them, and they rarely culminate in the occurrence of serious complications [4].
Antiviral drugs, such as acyclovir, are used to prevent the spread of the disease. Acyclovir is a synthetic analog of purine that is effective against HSV-1 and HSV-2. This drug functions by interfering with the DNA replication process and, therefore hindering the virus proliferation [5, 6]. The widespread use of oral acyclovir has led to the emergence of acyclovir-resistant species, inhibiting the therapeutic effect of acyclovir by mutations in their genome. Drug resistance in HSVs is a major concern in immunocompromised patients because they often need long-term antiviral therapy, which, in combination with persistent viral proliferation, heightens the risk of drug resistance. Since the level of resistance to such drug types is enhanced with such a mechanism, finding new treatment methods to eliminate the virus and also to hinder its proliferation, particularly in immunocompromised individuals, is a research priority [3, 7, 8].
Photodynamic therapy (PDT) is one of the therapeutic methods to confront viral infections. A wide variety of bacteria and viruses have shown susceptibility to PDT. PDT can influence intracellular activity such as cell metabolism, increase the adenosine triphosphate and blood flow, impair collagen synthesis, and decrease transcription factors. PDT is based on using a combination of three factors: a photosensitizer (PS), oxygen, and a certain wavelength of light [6, 9]. PDT is regarded as a totally non-invasive and selective method for patients. The PS is irradiated by a certain wavelength of light in presence of oxygen, resulting in generation of reactive oxygen species, which can result in cell death by damaging the proteins, nucleic acid, and lipid units. Reactive oxygen species are also unstable and are effective for a short period of time. Thus, finding a PS with the ability to selectively bind to the target virus-infected cells may improve the effectiveness of PDT [10, 11].
Different types of PSs are available, but the functional mechanism of all of which is based on two general principles. They either have a porphyrin ring in their structure or are categorized as non-porphyrin-based PSs. Methylene blue is one of the PSs used in PDT for therapeutic purposes [11]. Methylene blue, also known as methylthioninium chloride, is a derivative of hydrophilic phenothiazine. This substance is a PS used in 665 nm wavelength, which lies within the emission range of ordinary diode lasers and is used for low-level laser therapy [12].
Various in vitro and clinical studies used different radiation parameters to assess the impact of different laser intensities with different methylene blue concentrations (0.01%, 0.005%, and 0.0001%) or other PSs on acyclovir-sensitive HSV-1, and showed their effectiveness [6, 7, 9, 13-15]. However, no study has been conducted on the effect of PDT on acyclovir-resistant HSV-1. On the other hand, due to the increase in drug-resistant HSV-1 cases, this study was conducted to assess the effect of PDT with 660 nm diode laser with methylene blue PS with different concentrations and radiation parameters on acyclovir-resistant HSV-1.
Materials and Methods
Antiviral drugs, such as acyclovir, are used to prevent the spread of the disease. Acyclovir is a synthetic analog of purine that is effective against HSV-1 and HSV-2. This drug functions by interfering with the DNA replication process and, therefore hindering the virus proliferation [5, 6]. The widespread use of oral acyclovir has led to the emergence of acyclovir-resistant species, inhibiting the therapeutic effect of acyclovir by mutations in their genome. Drug resistance in HSVs is a major concern in immunocompromised patients because they often need long-term antiviral therapy, which, in combination with persistent viral proliferation, heightens the risk of drug resistance. Since the level of resistance to such drug types is enhanced with such a mechanism, finding new treatment methods to eliminate the virus and also to hinder its proliferation, particularly in immunocompromised individuals, is a research priority [3, 7, 8].
Photodynamic therapy (PDT) is one of the therapeutic methods to confront viral infections. A wide variety of bacteria and viruses have shown susceptibility to PDT. PDT can influence intracellular activity such as cell metabolism, increase the adenosine triphosphate and blood flow, impair collagen synthesis, and decrease transcription factors. PDT is based on using a combination of three factors: a photosensitizer (PS), oxygen, and a certain wavelength of light [6, 9]. PDT is regarded as a totally non-invasive and selective method for patients. The PS is irradiated by a certain wavelength of light in presence of oxygen, resulting in generation of reactive oxygen species, which can result in cell death by damaging the proteins, nucleic acid, and lipid units. Reactive oxygen species are also unstable and are effective for a short period of time. Thus, finding a PS with the ability to selectively bind to the target virus-infected cells may improve the effectiveness of PDT [10, 11].
Different types of PSs are available, but the functional mechanism of all of which is based on two general principles. They either have a porphyrin ring in their structure or are categorized as non-porphyrin-based PSs. Methylene blue is one of the PSs used in PDT for therapeutic purposes [11]. Methylene blue, also known as methylthioninium chloride, is a derivative of hydrophilic phenothiazine. This substance is a PS used in 665 nm wavelength, which lies within the emission range of ordinary diode lasers and is used for low-level laser therapy [12].
Various in vitro and clinical studies used different radiation parameters to assess the impact of different laser intensities with different methylene blue concentrations (0.01%, 0.005%, and 0.0001%) or other PSs on acyclovir-sensitive HSV-1, and showed their effectiveness [6, 7, 9, 13-15]. However, no study has been conducted on the effect of PDT on acyclovir-resistant HSV-1. On the other hand, due to the increase in drug-resistant HSV-1 cases, this study was conducted to assess the effect of PDT with 660 nm diode laser with methylene blue PS with different concentrations and radiation parameters on acyclovir-resistant HSV-1.
Materials and Methods
This in vitro, experimental study was conducted on HSV-1 strain KOS. This strain was identified by the polymerase chain reaction method and obtained from Tarbiat Modares University in Tehran. The study was ethically approved by the ethics committee of Islamic Azad University of Medical Sciences, Tehran (code of ethics: IR.IAU.DENTAL.REC.1401.082).
The sample size was calculated to be 5 in each group according to the results of a study by Svyatchenko et al, [16] and using the fixed effects ANOVA power analysis feature of SPSS 11, considering α=0.05, β=0.007 and effect size of 0.51 for methylene blue as the variable, and β=0.08 and effect size of 0.41 for laser energy density as the variable.
Virus proliferation and cell culture:
The viruses were proliferated through HeLa cells, which were obtained from the National Cell Bank of the Pasteur Institute of Iran. HeLa cells are epithelial cells of human origin derived from cervical cancer cells. HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (Biosera, France) supplemented with 10% fetal bovine serum (Biosera, England), 100 IU/mL penicillin G (Biosera, England), and 100 µg/mL streptomycin (Biosera, England), and incubated at 37°C in presence of 5% CO2. The infectious activity of the virus was examined using the tissue culture infectious dose 50 (TCID50) method as a standard approach for evaluation and titration of viruses. Hence, the effectiveness of the study's virucidal method was evaluated by this method, the titer was determined, and a specific amount of the virus suspension underwent radiation in different modes. After irradiation, each sample was individually titrated by the TCID50 method and compared with the control group [17].
Determination of HeLa cell-to-methylene blue toxicity threshold:
The methyl thiazolyl tetrazolium (MTT) assay was used to assess the cytotoxicity of methylene blue for HeLa cells. After adding different concentrations of methylene blue to a 96-well plate containing HeLa cells, the plate was incubated for 48 hours. Next, the MTT solution was added to all wells, and the plate was incubated again for 4 hours. The supernatant was removed, and dimethyl sulfoxide (LifeBiolab, Germany) was added to the wells and pipetted such that the formazan crystals were dissolved. Afterwards, the optical density (OD) of the wells was read by an ELISA reader (Biotek, USA) at 540 nm wavelength. The cytotoxicity was assessed using the following formula, and the CC50 value of methylene blue against HeLa cells was determined using the dose-response curve. CC50 is a concentration of a substance that destroys 50% of the cells [17, 18].
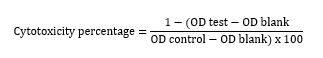
Test groups:
Diode laser was irradiated with 100 mW power in continuous wave mode with the cross-sectional area of the tip equal to 0.5 cm2 for 50, 100, and 150 seconds. The laser handpiece at the well opening was perpendicular to the suspension surface [19, 20]. The radiation doses employed in the current study were 10, 20, and 30 J/cm2.
The 20 study groups were as follows:
Groups 1 to 3: These groups involved the host cells and HSV-1, and were exposed to 660 nm laser irradiation with 100 mW power, and energy densities of 10, 20, and 30 J/cm2, with 50, 100, and 150 second irradiation times, respectively, without methylene blue.
Group 4: This group served as the control group and only contained cells and HSV-1, without radiation and without exposure to methylene blue.
Group 5 to 8: These groups involved the host cells and HSV-1, and were only exposed to methylene blue at 0.001%, 0.002%, 0.005%, and 0.01% concentrations, respectively.
Groups 9 to 11: These groups were exposed to 660 nm laser radiation with100 mW power, and 10, 20, and 30 J/cm2 energy densities with 50, 100, and 150 second irradiation times, respectively, in presence of 0.001% methylene blue.
Groups 12 to 14: These groups were exposed to 660 nm laser radiation with 100 mW power, and 10, 20, and 30 J/cm2 energy densities with 50, 100, and 150 second irradiation times, respectively, in presence of 0.002% methylene blue.
Groups 15 to 17: These groups were exposed to 660 nm laser radiation with 100 mW power, and 10, 20, and 30 J/cm2 energy densities with 50, 100, and 150 second irradiation times, respectively, in presence of 0.005% methylene blue.
Groups 18 to 20: These groups were exposed to 660 nm laser radiation with 100 mW power, and 10, 20, and 30 J/cm2 energy densities with 50, 100, and 150 second irradiation times, in presence of 0.01% methylene blue.
TCID50:
The HeLa cells were cultured in 96-well plates and infected with HSV-1 prepared dilutions. The infected plate was incubated for one hour in a CO2 incubator at 37°C. The cells were then washed with phosphate buffered saline, and Dulbecco’s modified Eagle’s medium was added to all wells.
The 96-well plate was incubated at 37°C in presence of 5% CO2 and assessed daily for presence of the cytopathic effect. The cytopathic effect criterion for HSV-1 was in the form of rounding and enlargement of the infected cells. The final result was recorded after 72 hours, and the virus TCID50 was measured using the Reed and Muench method (calculation formula for obtaining the TCID50 result) [18].
SPSS software version 26 and two- way ANOVA were used for data analysis. P values smaller than 0.05 were considered statistically significant.
Results
The sample size was calculated to be 5 in each group according to the results of a study by Svyatchenko et al, [16] and using the fixed effects ANOVA power analysis feature of SPSS 11, considering α=0.05, β=0.007 and effect size of 0.51 for methylene blue as the variable, and β=0.08 and effect size of 0.41 for laser energy density as the variable.
Virus proliferation and cell culture:
The viruses were proliferated through HeLa cells, which were obtained from the National Cell Bank of the Pasteur Institute of Iran. HeLa cells are epithelial cells of human origin derived from cervical cancer cells. HeLa cells were cultured in Dulbecco’s modified Eagle’s medium (Biosera, France) supplemented with 10% fetal bovine serum (Biosera, England), 100 IU/mL penicillin G (Biosera, England), and 100 µg/mL streptomycin (Biosera, England), and incubated at 37°C in presence of 5% CO2. The infectious activity of the virus was examined using the tissue culture infectious dose 50 (TCID50) method as a standard approach for evaluation and titration of viruses. Hence, the effectiveness of the study's virucidal method was evaluated by this method, the titer was determined, and a specific amount of the virus suspension underwent radiation in different modes. After irradiation, each sample was individually titrated by the TCID50 method and compared with the control group [17].
Determination of HeLa cell-to-methylene blue toxicity threshold:
The methyl thiazolyl tetrazolium (MTT) assay was used to assess the cytotoxicity of methylene blue for HeLa cells. After adding different concentrations of methylene blue to a 96-well plate containing HeLa cells, the plate was incubated for 48 hours. Next, the MTT solution was added to all wells, and the plate was incubated again for 4 hours. The supernatant was removed, and dimethyl sulfoxide (LifeBiolab, Germany) was added to the wells and pipetted such that the formazan crystals were dissolved. Afterwards, the optical density (OD) of the wells was read by an ELISA reader (Biotek, USA) at 540 nm wavelength. The cytotoxicity was assessed using the following formula, and the CC50 value of methylene blue against HeLa cells was determined using the dose-response curve. CC50 is a concentration of a substance that destroys 50% of the cells [17, 18].
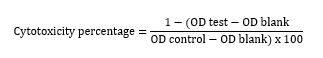
Test groups:
Diode laser was irradiated with 100 mW power in continuous wave mode with the cross-sectional area of the tip equal to 0.5 cm2 for 50, 100, and 150 seconds. The laser handpiece at the well opening was perpendicular to the suspension surface [19, 20]. The radiation doses employed in the current study were 10, 20, and 30 J/cm2.
The 20 study groups were as follows:
Groups 1 to 3: These groups involved the host cells and HSV-1, and were exposed to 660 nm laser irradiation with 100 mW power, and energy densities of 10, 20, and 30 J/cm2, with 50, 100, and 150 second irradiation times, respectively, without methylene blue.
Group 4: This group served as the control group and only contained cells and HSV-1, without radiation and without exposure to methylene blue.
Group 5 to 8: These groups involved the host cells and HSV-1, and were only exposed to methylene blue at 0.001%, 0.002%, 0.005%, and 0.01% concentrations, respectively.
Groups 9 to 11: These groups were exposed to 660 nm laser radiation with100 mW power, and 10, 20, and 30 J/cm2 energy densities with 50, 100, and 150 second irradiation times, respectively, in presence of 0.001% methylene blue.
Groups 12 to 14: These groups were exposed to 660 nm laser radiation with 100 mW power, and 10, 20, and 30 J/cm2 energy densities with 50, 100, and 150 second irradiation times, respectively, in presence of 0.002% methylene blue.
Groups 15 to 17: These groups were exposed to 660 nm laser radiation with 100 mW power, and 10, 20, and 30 J/cm2 energy densities with 50, 100, and 150 second irradiation times, respectively, in presence of 0.005% methylene blue.
Groups 18 to 20: These groups were exposed to 660 nm laser radiation with 100 mW power, and 10, 20, and 30 J/cm2 energy densities with 50, 100, and 150 second irradiation times, in presence of 0.01% methylene blue.
TCID50:
The HeLa cells were cultured in 96-well plates and infected with HSV-1 prepared dilutions. The infected plate was incubated for one hour in a CO2 incubator at 37°C. The cells were then washed with phosphate buffered saline, and Dulbecco’s modified Eagle’s medium was added to all wells.
The 96-well plate was incubated at 37°C in presence of 5% CO2 and assessed daily for presence of the cytopathic effect. The cytopathic effect criterion for HSV-1 was in the form of rounding and enlargement of the infected cells. The final result was recorded after 72 hours, and the virus TCID50 was measured using the Reed and Muench method (calculation formula for obtaining the TCID50 result) [18].
SPSS software version 26 and two- way ANOVA were used for data analysis. P values smaller than 0.05 were considered statistically significant.
Results
The result of methylene blue cytotoxicity for HeLa cells by the MTT assay is shown in Figure 1. The CC50 of methylene blue for HeLa cells after 48 hours was 15.40 µg/mL, indicating that none of the tested methylene blue concentrations had a cytotoxic effect on HeLa cells.
Figure 1. Methylene blue toxicity for HeLa cells after 48 hours
The HSV-1 titer in the study groups is presented in Table 1.
Two-way ANOVA showed that the effects of methylene blue concentration (P=0.675), and laser energy density (P=0.914), and their interaction effect (P=0.977) were not significant on the titer of acyclovir-resistant HSV-1.
The HSV-1 titer in the study groups is presented in Table 1.
Two-way ANOVA showed that the effects of methylene blue concentration (P=0.675), and laser energy density (P=0.914), and their interaction effect (P=0.977) were not significant on the titer of acyclovir-resistant HSV-1.
The current results demonstrated that different methylene blue concentrations (0.001%, 0.002%, 0.005%, and 0.01%), 660 nm laser energy densities (10, 20, and 30 J/cm2), and their interaction had no significant effect on the titer of acyclovir-resistant HSV-1. Latief et al. [7] evaluated the impact of antimicrobial PDT (660 nm, 10-30 J/cm2) with TONS 504 [13,17-bis(1-carboxyyethyl) carbamoyl (3-methylpyridine)-3-(1,3-dioxane-2-yl)] (0.01 to 10 mg/L) on human fibroblast cells infected with acyclovir-resistant and acyclovir-sensitive HSV-1 strains.
PDT, together with TONS 504 with 10 mg/L concentration completely eliminated both acyclovir-resistant and acyclovir-sensitive HSV-1 strains. Difference between their results and the present findings may be attributed to using different PSs. Latief et al. [7] reported no significant effect in absence of TONS 504 or PDT light source, which was consistent with the present results. Another study also found that PDT with 660 nm laser (with 30 J/cm2 and 60 J/cm2 energy densities) had no effect on HSV-1 in monkey kidney cells [21], which was somewhat in line with the present findings.
Unlike the current findings, Namvar et al, [6] in their in vitro study found that PDT with 810 nm laser and indocyanine green resulted in a significant decrease in herpes labialis viral titer. In a systematic review, Khalil and Hamadah [13] assessed the effect of PDT on herpes labialis and found that PDT could treat vesicular herpes labialis. In another systematic review, Lotufo et al. [14] investigated the impact of PDT on herpes labialis and found that PDT (630-660 nm laser with 100-120 J/cm2 energy density) with methylene blue and 5-aminolevulinic acid was effective for treatment of herpes labialis. Different parameters of PDT, such as different wavelengths and PSs, evaluation of acyclovir-resistant HSV-1 strain, and the distance of the laser tip from the medium surface can explain inconsistencies in the reported results.
In a clinical trial, Zanella et al. [15] reported that PDT with 808 nm laser (150 mW, 22.22-11.11 J/cm2 1-2 J at point) and methylene blue (0.01% concentration) relieved pain in patients with recurrent herpes labialis. In another clinical trial, Ajmal [9] reported that PDT with 660 nm laser (150 mW, with a total energy of 300 J/cm2, 4.5 J at each point for 60 seconds) and methylene blue (0.005% concentration) along with 5% topical acyclovir controlled pain in patients with vesicular HSV-1. Ramalho et al, [5] in their clinical trial demonstrated that PDT with 660 nm laser (150 mW, with a total energy of 40 J/cm, 4.8 J at each point for 120 seconds) and methylene blue (0.005% concentration) decreased the wound size, edema, and tingling in patients with HSV-1. In a case report, Lago et al. [22] reported that PDT with 660 nm laser (100 mW, 100 J/cm2, 3 J at each point for 30 seconds) and methylene blue (0.01% concentration) relieved pain and discomfort in three patients with HSV-1. Ramalho et al. [23] treated two patients with herpes labialis in macular and vesicular phases using PDT (660 nm, 120 J/cm2, for 2 minutes at each point) with methylene blue (0.005%). In patient number 1, PDT expedited the vesicular phase, and in patient number 2, a combination of acyclovir and PDT inhibited the spread of infection. The observed differences can be due to differences in study designs, laser parameters, type and concentration of PSs, evaluation of acyclovir-resistant HSV-1 strain, and distance from the laser tip and the medium surface.
Marotti et al. [24] used PDT with 0.01% methylene blue for 5 minutes in 4 patients with HSV lesions in the vesicular phase. The lesions were subjected to continuous laser irradiation with 660 nm wavelength and 0.04 cm2 spot size. The laser power was 40 mW, and was irradiated for 2 minutes at each point, with 4.8 J energy (total energy 19.2 J). The same wavelength was used after 24 hours, 72 hours, and 1 week, with an energy density of 3.8 J/cm2. No discomfort was reported during treatment, and the 6-month follow-up period was associated with satisfactory outcomes. In a case report, Marotti et al. [25] used PDT with 0.001% methylene blue and 660 nm laser with 100 J/cm2 energy density in two patients with herpes lesions who were in the vesicular phase and reported optimal results. Variations in laser parameters can explain the controversy in the results. Moreover, the above-mentioned studies were conducted clinically on the acyclovir-sensitive strain, while the current study was conducted in vitro on an acyclovir-resistant strain.
Oral herpes lesions are painful and recurrent, causing discomfort and an unesthetic appearance. The gold standard treatment is acyclovir, which is the most common medication used to inhibit or suppress these lesions [26]; however, continuous use of this drug gives rise to viral drug resistance [9, 25]. Hence, PDT can be employed as an alternative or adjunctive treatment in herpetic lesions. In PDT, the PS absorbs a certain wavelength of light and converts it into useful energy, which leads to production of cytotoxic agents such as reactive oxygen species [6, 9]. Therefore, in PDT, reactive oxygen is generated by the PS, and low-intensity light is used to kill the infectious cells [9, 26]. Methylene blue has already been described as a safe and influential PS for use in PDT for antimicrobial purposes; this method has been proven to be influential for inactivation of enveloped and non-enveloped viruses [27]. Methylene blue specifically works by damaging the viral nucleic acid; in other words, the reaction of methylene blue with red laser light can break the viral DNA into pieces. Another advantage of PDT is lack of complications. No complications have been reported for this method or for methylene blue [5].
Although oral acyclovir is typically prescribed for patients experiencing 6 or more relapses annually, patients with fewer relapses can only rely on topical acyclovir as a treatment option. However, topical acyclovir is still widely used by patients experiencing recurrent lesions, and because of the absence of other efficient treatments, oral antiviral drugs can only be used in severe cases due to virus resistance to this treatment [5].
It should also be noted that the effectiveness of PDT for HSV-1 may depend on various factors such as laser parameters (wavelength, energy density, duration of irradiation) and target tissue (chromophore, structure, thickness) [15]. PDT with different energy densities of 660 nm laser and different methylene blue concentrations applied in this study did not decrease the acyclovir-resistant HSV-1 viral titer.
Evaluation of the acyclovir-resistant strain and different laser energy densities was the main strength of this study. This study was conducted in vitro, which is a considerable limitation, and the results should be interpreted with caution. In vitro and clinical studies are recommended to assess the efficacy of PDT with higher energy densities along with other PSs in different concentrations for elimination of acyclovir-resistant HSV-1.
Conclusion
PDT, together with TONS 504 with 10 mg/L concentration completely eliminated both acyclovir-resistant and acyclovir-sensitive HSV-1 strains. Difference between their results and the present findings may be attributed to using different PSs. Latief et al. [7] reported no significant effect in absence of TONS 504 or PDT light source, which was consistent with the present results. Another study also found that PDT with 660 nm laser (with 30 J/cm2 and 60 J/cm2 energy densities) had no effect on HSV-1 in monkey kidney cells [21], which was somewhat in line with the present findings.
Unlike the current findings, Namvar et al, [6] in their in vitro study found that PDT with 810 nm laser and indocyanine green resulted in a significant decrease in herpes labialis viral titer. In a systematic review, Khalil and Hamadah [13] assessed the effect of PDT on herpes labialis and found that PDT could treat vesicular herpes labialis. In another systematic review, Lotufo et al. [14] investigated the impact of PDT on herpes labialis and found that PDT (630-660 nm laser with 100-120 J/cm2 energy density) with methylene blue and 5-aminolevulinic acid was effective for treatment of herpes labialis. Different parameters of PDT, such as different wavelengths and PSs, evaluation of acyclovir-resistant HSV-1 strain, and the distance of the laser tip from the medium surface can explain inconsistencies in the reported results.
In a clinical trial, Zanella et al. [15] reported that PDT with 808 nm laser (150 mW, 22.22-11.11 J/cm2 1-2 J at point) and methylene blue (0.01% concentration) relieved pain in patients with recurrent herpes labialis. In another clinical trial, Ajmal [9] reported that PDT with 660 nm laser (150 mW, with a total energy of 300 J/cm2, 4.5 J at each point for 60 seconds) and methylene blue (0.005% concentration) along with 5% topical acyclovir controlled pain in patients with vesicular HSV-1. Ramalho et al, [5] in their clinical trial demonstrated that PDT with 660 nm laser (150 mW, with a total energy of 40 J/cm, 4.8 J at each point for 120 seconds) and methylene blue (0.005% concentration) decreased the wound size, edema, and tingling in patients with HSV-1. In a case report, Lago et al. [22] reported that PDT with 660 nm laser (100 mW, 100 J/cm2, 3 J at each point for 30 seconds) and methylene blue (0.01% concentration) relieved pain and discomfort in three patients with HSV-1. Ramalho et al. [23] treated two patients with herpes labialis in macular and vesicular phases using PDT (660 nm, 120 J/cm2, for 2 minutes at each point) with methylene blue (0.005%). In patient number 1, PDT expedited the vesicular phase, and in patient number 2, a combination of acyclovir and PDT inhibited the spread of infection. The observed differences can be due to differences in study designs, laser parameters, type and concentration of PSs, evaluation of acyclovir-resistant HSV-1 strain, and distance from the laser tip and the medium surface.
Marotti et al. [24] used PDT with 0.01% methylene blue for 5 minutes in 4 patients with HSV lesions in the vesicular phase. The lesions were subjected to continuous laser irradiation with 660 nm wavelength and 0.04 cm2 spot size. The laser power was 40 mW, and was irradiated for 2 minutes at each point, with 4.8 J energy (total energy 19.2 J). The same wavelength was used after 24 hours, 72 hours, and 1 week, with an energy density of 3.8 J/cm2. No discomfort was reported during treatment, and the 6-month follow-up period was associated with satisfactory outcomes. In a case report, Marotti et al. [25] used PDT with 0.001% methylene blue and 660 nm laser with 100 J/cm2 energy density in two patients with herpes lesions who were in the vesicular phase and reported optimal results. Variations in laser parameters can explain the controversy in the results. Moreover, the above-mentioned studies were conducted clinically on the acyclovir-sensitive strain, while the current study was conducted in vitro on an acyclovir-resistant strain.
Oral herpes lesions are painful and recurrent, causing discomfort and an unesthetic appearance. The gold standard treatment is acyclovir, which is the most common medication used to inhibit or suppress these lesions [26]; however, continuous use of this drug gives rise to viral drug resistance [9, 25]. Hence, PDT can be employed as an alternative or adjunctive treatment in herpetic lesions. In PDT, the PS absorbs a certain wavelength of light and converts it into useful energy, which leads to production of cytotoxic agents such as reactive oxygen species [6, 9]. Therefore, in PDT, reactive oxygen is generated by the PS, and low-intensity light is used to kill the infectious cells [9, 26]. Methylene blue has already been described as a safe and influential PS for use in PDT for antimicrobial purposes; this method has been proven to be influential for inactivation of enveloped and non-enveloped viruses [27]. Methylene blue specifically works by damaging the viral nucleic acid; in other words, the reaction of methylene blue with red laser light can break the viral DNA into pieces. Another advantage of PDT is lack of complications. No complications have been reported for this method or for methylene blue [5].
Although oral acyclovir is typically prescribed for patients experiencing 6 or more relapses annually, patients with fewer relapses can only rely on topical acyclovir as a treatment option. However, topical acyclovir is still widely used by patients experiencing recurrent lesions, and because of the absence of other efficient treatments, oral antiviral drugs can only be used in severe cases due to virus resistance to this treatment [5].
It should also be noted that the effectiveness of PDT for HSV-1 may depend on various factors such as laser parameters (wavelength, energy density, duration of irradiation) and target tissue (chromophore, structure, thickness) [15]. PDT with different energy densities of 660 nm laser and different methylene blue concentrations applied in this study did not decrease the acyclovir-resistant HSV-1 viral titer.
Evaluation of the acyclovir-resistant strain and different laser energy densities was the main strength of this study. This study was conducted in vitro, which is a considerable limitation, and the results should be interpreted with caution. In vitro and clinical studies are recommended to assess the efficacy of PDT with higher energy densities along with other PSs in different concentrations for elimination of acyclovir-resistant HSV-1.
Conclusion
In this in vitro study, PDT with different energy densities (10, 20, and 30 J/cm2) of 660 nm laser and different methylene blue concentrations (0.001%, 0.002%, 0.005%, and 0.01%) could not decrease the acyclovir-resistant HSV-1 titer.
Type of Study: Original article |
Subject:
Oral medicine
References
1. Monjo AL, Pringle ES, Thornbury M, Duguay BA, Monro SMA, Hetu M, et al. Photodynamic Inactivation of Herpes Simplex Viruses. Viruses. 2018 Sep 29;10(10):532. [DOI:10.3390/v10100532] [PMID] []
2. Teitelbaum S, Azevedo LH, Bernaola-Paredes WE. Antimicrobial Photodynamic Therapy Used as First Choice to Treat Herpes Zoster Virus Infection in Younger Patient: A Case Report. Photobiomodul Photomed Laser Surg. 2020 Apr;38(4):232-6. [DOI:10.1089/photob.2019.4725] [PMID]
3. Wouk J, Celestino GG, Rodrigues BCD, Malfatti CRM, Cunha MAA, Orsato A, et al. Sulfonated (1 → 6)-β-d-Glucan (Lasiodiplodan): A Promising Candidate against the Acyclovir-Resistant Herpes Simplex Virus Type 1 (HSV-1) Strain. Biomacromolecules. 2022 Oct 10;23(10):4041-52. [DOI:10.1021/acs.biomac.2c00156] [PMID]
4. Malary M, Abedi G, Hamzehgardeshi Z, Afshari M, Moosazadeh M. The prevalence of herpes simplex virus type 1 and 2 infection in Iran: A meta-analysis. Int J Reprod Biomed. 2016 Oct;14(10):615-24. [DOI:10.29252/ijrm.14.10.615] [PMID]
5. Ramalho KM, Cunha SR, Gonçalves F, Escudeiro GS, Steiner-Oliveira C, Horliana ACRT, et al. Photodynamic therapy and Acyclovir in the treatment of recurrent herpes labialis: A controlled randomized clinical trial. Photodiagnosis Photodyn Ther. 2021 Mar;33:102093. [DOI:10.1016/j.pdpdt.2020.102093] [PMID]
6. Namvar MA, Vahedi M, Abdolsamadi HR, Mirzaei A, Mohammadi Y, Azizi Jalilian F. Effect of photodynamic therapy by 810 and 940 nm diode laser on Herpes Simplex Virus 1: An in vitro study. Photodiagnosis Photodyn Ther. 2019 Mar;25:87-91. [DOI:10.1016/j.pdpdt.2018.11.011] [PMID]
7. Latief MA, Chikama T, Ko JA, Kiuchi Y, Sakaguchi T, Obana A. Inactivation of acyclovir-sensitive and -resistant strains of herpes simplex virus type 1 in vitro by photodynamic antimicrobial chemotherapy. Mol Vis. 2015 May 2;21:532-7.
8. Schalkwijk HH, Snoeck R, Andrei G. Acyclovir resistance in herpes simplex viruses: Prevalence and therapeutic alternatives. Biochem Pharmacol. 2022 Dec;206:115322. [DOI:10.1016/j.bcp.2022.115322] [PMID]
9. Ajmal M. Effectiveness of photodynamic therapy as an adjunct to topical antiviral therapy in the treatment of herpes labialis: A randomized controlled clinical trial. Photodiagnosis Photodyn Ther. 2021 Jun;34:102302. [DOI:10.1016/j.pdpdt.2021.102302] [PMID]
10. Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011 Jul-Aug;61(4):250-81. [DOI:10.3322/caac.20114] [PMID] []
11. Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. 2016 Feb 15;473(4):347-64. [DOI:10.1042/BJ20150942] [PMID] []
12. Wainwright M, Crossley KB. Methylene Blue--a therapeutic dye for all seasons? J Chemother. 2002 Oct;14(5):431-43. [DOI:10.1179/joc.2002.14.5.431] [PMID]
13. Khalil M, Hamadah O. Association of Photodynamic Therapy and Photobiomodulation As a Promising Treatment of Herpes Labialis: A Systematic Review. Photobiomodul Photomed Laser Surg. 2022 May;40(5):299-307. [DOI:10.1089/photob.2021.0186] [PMID]
14. Lotufo MA, Tempestini Horliana ACR, Santana T, de Queiroz AC, Gomes AO, Motta LJ, et al. Efficacy of photodynamic therapy on the treatment of herpes labialis: A systematic review. Photodiagnosis Photodyn Ther. 2020 Mar;29:101536. [DOI:10.1016/j.pdpdt.2019.08.018] [PMID]
15. Zanella PA, Onuchic LF, Watanabe EH, Azevedo LH, Aranha ACC, Ramalho KM, et al. Photobiomodulation for Preventive Therapy of Recurrent Herpes Labialis: A 2-Year In Vivo Randomized Controlled Study. Photobiomodul Photomed Laser Surg. 2022 Oct;40(10):682-90. [DOI:10.1089/photob.2022.0054] [PMID] []
16. Svyatchenko VA, Nikonov SD, Mayorov AP, Gelfond ML, Loktev VB. Antiviral photodynamic therapy: Inactivation and inhibition of SARS-CoV-2 in vitro using methylene blue and Radachlorin. Photodiagnosis Photodyn Ther. 2021 Mar;33:102112. [DOI:10.1016/j.pdpdt.2020.102112] [PMID] []
17. Ebrahimi E, Mousavi-Jazayeri SM, Rezaee MB, Parsania M. Antiviral effects of Aloe vera (L.) Burm. f. and Ruta graveolens L. extract on acyclovir-resistant herpes simplex virus type 1. Journal of Medicinal plants and By-Products. 2021 Apr 1;10(1):103-8.
18. Parsania A, Pouriayevali MH, Parsania M, Ghorbani M. Chelidonium majus L. alkaloid extract enhances TRAIL-induced apoptosis in HeLa cell line through death receptors 4 and 5 upregulation. Gene Reports. 2021 Dec 1;25:101311. [DOI:10.1016/j.genrep.2021.101311]
19. Al-Maweri SA, Kalakonda B, AlAizari NA, Al-Soneidar WA, Ashraf S, Abdulrab S, et al. Efficacy of low-level laser therapy in management of recurrent herpes labialis: a systematic review. Lasers Med Sci. 2018 Sep;33(7):1423-30. [DOI:10.1007/s10103-018-2542-5] [PMID]
20. Sampaio LS, de Annunzio SR, de Freitas LM, Dantas LO, de Boni L, Donatoni MC, et al. Influence of light intensity and irradiation mode on methylene blue, chlorin-e6 and curcumin-mediated photodynamic therapy against Enterococcus faecalis. Photodiagnosis Photodyn Ther. 2020 Sep;31:101925. [DOI:10.1016/j.pdpdt.2020.101925] [PMID]
21. Pinheiro RMC, Romanos MTV, Canabarro A, Aranha AC, Prado M, de Carvalho Ferreira D, et al. The effects of photodynamic therapy on Vero cells and replication of herpes simplex type 1: an in vitro analysis. Lasers Dent Sci. 2021; 5: 223-28.22. [DOI:10.1007/s41547-021-00138-7]
22. Lago ADN, Fortes ABC, Furtado GS, Menezes CFS, Gonçalves LM. Association of antimicrobial photodynamic therapy and photobiomodulation for herpes simplex labialis resolution: Case series. Photodiagnosis Photodyn Ther. 2020 Dec;32:102070. [DOI:10.1016/j.pdpdt.2020.102070] [PMID]
23. Ramalho KM, Rocha RG, Correa-Aranha AC, Cunha SR, Simões A, Campos L, et al. Treatment of herpes simplex labialis in macule and vesicle phases with photodynamic therapy. Report of two cases. Photodiagnosis Photodyn Ther. 2015 Jun;12(2):321-3. [DOI:10.1016/j.pdpdt.2015.02.005] [PMID]
24. Marotti J, Aranha AC, Eduardo Cde P, Ribeiro MS. Photodynamic therapy can be effective as a treatment for herpes simplex labialis. Photomed Laser Surg. 2009 Apr;27(2):357-63. [DOI:10.1089/pho.2008.2268] [PMID]
25. Marotti J, Sperandio FF, Fregnani ER, Aranha AC, de Freitas PM, Eduardo Cde P. High-intensity laser and photodynamic therapy as a treatment for recurrent herpes labialis. Photomed Laser Surg. 2010 Jun;28(3):439-44. [DOI:10.1089/pho.2009.2522] [PMID]
26. La Selva A, Negreiros RM, Bezerra DT, Rosa EP, Pavesi VCS, et al. Treatment of herpes labialis by photodynamic therapy: Study protocol clinical trial (SPIRIT compliant). Medicine (Baltimore). 2020 Mar;99(12):e19500. [DOI:10.1097/MD.0000000000019500] [PMID] []
27. de Paula Eduardo C, Aranha AC, Simões A, Bello-Silva MS, Ramalho KM, Esteves-Oliveira M, et al. Laser treatment of recurrent herpes labialis: a literature review. Lasers Med Sci. 2014 Jul;29(4):1517-29. [DOI:10.1007/s10103-013-1311-8]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







