Volume 10, Issue 2 (6-2025)
J Res Dent Maxillofac Sci 2025, 10(2): 152-158 |
Back to browse issues page
Ethics code: IR.TUMS.DENTISTRY.REC.1398.122
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Motevaselian F, Mohaghegh B, Sadeghi F, Rafeie N, Abbasi M, Ranjbar Omrani L. Dentin Remineralizing Potential of a Bioactive Composite Resin Versus a Conventional Composite Resin: Mineral Index and Microhardness Analysis. J Res Dent Maxillofac Sci 2025; 10 (2) :152-158
URL: http://jrdms.dentaliau.ac.ir/article-1-764-en.html
URL: http://jrdms.dentaliau.ac.ir/article-1-764-en.html
Fariba Motevaselian1 
 , Behnam Mohaghegh2
, Behnam Mohaghegh2 
 , Farzaneh Sadeghi1
, Farzaneh Sadeghi1 
 , Niyousha Rafeie3
, Niyousha Rafeie3 
 , Mehdi Abbasi *4
, Mehdi Abbasi *4 
 , Ladan Ranjbar Omrani5
, Ladan Ranjbar Omrani5 


 , Behnam Mohaghegh2
, Behnam Mohaghegh2 
 , Farzaneh Sadeghi1
, Farzaneh Sadeghi1 
 , Niyousha Rafeie3
, Niyousha Rafeie3 
 , Mehdi Abbasi *4
, Mehdi Abbasi *4 
 , Ladan Ranjbar Omrani5
, Ladan Ranjbar Omrani5 

1- Restorative Department, Dental School, Tehran University of Medical Sciences, Tehran, Iran.
2- Dentist, Private practice, Tehran, Iran.
3- Dental Research Center, Dentistry Research Institute, Dental School, Tehran University of Medical Science, Tehran, Iran.
4- Restorative Department, Dental School, Tehran University of Medical Sciences, Tehran, Iran. ,Abbasimahdi84@gmail.com
5- RRestorative Department, Dental School, Tehran University of Medical Sciences, Tehran, Iran.
2- Dentist, Private practice, Tehran, Iran.
3- Dental Research Center, Dentistry Research Institute, Dental School, Tehran University of Medical Science, Tehran, Iran.
4- Restorative Department, Dental School, Tehran University of Medical Sciences, Tehran, Iran. ,
5- RRestorative Department, Dental School, Tehran University of Medical Sciences, Tehran, Iran.
Keywords: Activa Bioactive Base Liner, Activa Bioactive Restorative, Flowable Hybrid Composite, Tooth Remineralization
Full-Text [PDF 333 kb]
(848 Downloads)
| Abstract (HTML) (1573 Views)
Full-Text: (399 Views)
Abstract
Background and Aim: This study aimed to compare the dentin demineralization inhibitory potential of a bioactive composite resin (ACTIVA-BioActive) with a conventional composite resin.
Materials and Methods: In this in vitro experimental study, 45 class V cavities were prepared on the root surface of extracted human third molars. The teeth were then immersed in a demineralizing solution (lactic acid, pH=4.5) at 37˚C for 3 days to induce the formation of secondary caries. The cavities were restored with Single bond 2 + Z250 (group A), ACTIVA BioActive (group B), and Single bond 2 + ACTIVA BioActive (group C). The dentin microhardness was measured close to the restoration margin (marginally exposed dentin), and at approximately 4 mm distance from the margin in a varnish-covered dentin area (protected dentin). Three measurements were made at each site at 50, 100, and 150 µm depths from the external dentin surface. Data were analyzed with one-way ANOVA and Tukey test (alpha=0.05).
Results: The highest Vickers hardness number (VHN) was observed in ACTIVA+ bonding agent (49.46±4.15), followed by Z250 (44.57±3.87), and ACTIVA (43.44±3.76) group. The mineral index was significantly higher in ACTIVA+ bonding agent (76.87±3.80) compared to other groups while no significant difference was observed between ACTIVA (70.85±4.06) and Z250 (71.98±3.09) in this regard (P<0.001).
Conclusion: The results showed that flowable ACTIVA BioActive composite used with Single bond 2 resulted in a significantly smaller reduction in dentin microhardness and reduced secondary caries formation. ACTIVA BioActive with no adhesive had no advantage over Z250 composite.
Keywords: Activa Bioactive Base Liner; Activa Bioactive Restorative; Flowable Hybrid Composite; Tooth Remineralization
Introduction
Dental caries is one of the most common infectious diseases around the world [1]. In recent years, resin restorative materials have been widely used due to their optimal esthetic properties and easy application in the clinical setting [2,3]. Despite many advances in composite resin formulations to improve physical properties, many composite resin materials still contain Bis-GMA, which is responsible for polymerization shrinkage during the setting reaction [4,5]. This shrinkage causes stress, which subsequently results in gap formation, enamel microcracks, microleakage, secondary caries, tooth hypersensitivity, and discoloration around the restoration margins [6]. This highlights the need for developing effective antibacterial and bioactive restorative materials that can prevent bacterial colonization and secondary caries [7], subsequently increasing the longevity of composite restorations [8].
In recent years, a new generation of restorative materials known as bioactive composite resins was introduced to dental market. These materials can form chemical bonds to tooth structure without adhesive agents. Absence of monomers responsible for polymerization shrinkage in the composition of bioactive composite resins prevents further shrinkage and stress during polymerization process. Additionally, silver bioglass fillers and amorphous phosphopeptide calcium phosphate present in their composition act as a scaffold or matrix for further remineralization process [9,10]. In a study conducted by Chatzistavrou et al. [7], a composite resin containing silver bioglass enhanced the remineralization properties by increasing the formation of calcium-phosphate apatite-like phase in field emission scanning electron microscopy analysis after 14 days. In addition, these materials have both self-cure and light-cure setting modes, which decrease the concerns regarding the curing depth and microleakage [9,10].
ACTIVA-BioActive is one of the newly introduced bioactive materials. In fact, ACTIVA- BioActive is a hydrophilic resin-modified glass ionomer cement enriched with bioglass and fortified with a patented rubberized polymer resin [11]. The ion release, microleakage, and bond strength of ACTIVA composite resin have been previously evaluated with reportedly promising results. Porenczuk et al. [11] reported fluoride release from ACTIVA composite, which was the highest in the first 24 hours. Zmener et al. [12] reported significantly higher flexural strength and flexural fatigue of ACTIVA composite compared to conventional glass ionomer cement and flowable composite resin. However, the authors did not find any study evaluating the effect of ACTIVA-BioActive composite resin as a bioactive material on microhardness of the adjacent dentin. Thus, due to the limited information regarding the effect of this material on the hardness of adjacent dentin and its remineralization potential, the present study aimed to compare the microhardness of dentin adjacent to ACTIVA composite with and without a bonding agent, and Z250 composite resin. The null hypothesis was that the microhardness values of dentin adjacent to ACTIVA with and without a bonding agent and Z-250 composite would not be significantly different.
Materials and Methods
This in vitro experimental study was approved by the ethics committee of Tehran University of Medical Sciences (IR.TUMS.DENTISTRY. REC.1398.122).
Sample size:
The minimum sample size was calculated to be 15 specimens in each experimental group using one-way ANOVA feature of PASS 11 (NCSS, LLC., Kaysville, Utah, USA), considering alpha=0.05, beta=0.2, mean standard deviation of microhardness equal to 4.97, and effect size of 0.49 [13].
Sample preparation:
Forty-five freshly extracted human third molars were used in the present study. The teeth had no caries, physical anomaly, or crack, and were stored in saline solution at 4°C until use. After removing the residual soft tissue and calculus, the teeth were stored in 0.5% chloramine T (Wako Pure Chemical Industry, Osaka, Japan) for one week for disinfection. To remove cement layer from the root surface, the samples were polished with 1000-grit silicon carbide paper (Sof-Lex, 3M ESPE, USA) under water irrigation.
Each sample was split in half in mesiodistal direction to obtain two symmetrical halves. The samples were then randomly divided into 3 groups (n=15). Standard class V cavities (2.5 mm in diameter and 1 mm in depth) were prepared on the samples 1 mm below the cementoenamel junction using a fine diamond bur (Intensiv #2201, INTENSIV SA, Switzerland) in a high-speed handpiece under water coolant [14]. The bur used in the handpiece was changed after each 5 preparations. Afterwards, the prepared cavities were cleaned using pumice slurry. Then, each sample was restored according to its group as follows:
Z-250: The samples were etched with 37% phosphoric acid (Scotchbond, 3M ESPE, USA) for 15 seconds, followed by 10 seconds of rinsing with water. Then, a cotton pellet was used to remove excess water from each cavity. The bonding agent (Single bond, 3M ESPE, USA) was applied in each cavity with a microbrush and was dried with gentle air flow for 10 seconds. The second layer of bonding agent was applied with the same approach. The samples were cured using a polywave LED curing unit (Woodpecker LED Curing, Guilin Woodpecker Medical Instrument Co., Guilin, China) with 1000 mW/cm2 power intensity for 20 seconds. Next, one layer of composite resin (Z250; 3M ESPE, MN, USA) was placed in the cavity and light-cured for 40 seconds.
ACTIVA: No etching or adhesive agent was used. Flowable ACTIVA™ BioActive composite resin (Pulpdent, Watertown, MA, USA) was placed in the cavities and light-cured for 20 seconds.
ACTIVA+ bonding agent: The samples were etched, and the bonding agent was used in the same manner as explained in group A. Then, ACTIVA™ BioActive flowable composite was placed in the cavities and light-cured for 20 seconds.
Finally, the cavity margins were polished using 600-, 800-, 1000-, and 1200-grit polishing discs (3M, St. Paul, MN, USA) under water spray and rinsed with deionized water. All surfaces of the samples except the composite surface and 1 mm dentin adjacent to the composite were covered with 2 layers of acid-resistant varnish. The samples were then subjected to thermocycling in water baths between 5°C and 55°C with a dwell time of 15 seconds for 10,000 cycles corresponding to one year of clinical performance [15].
Demineralization process:
The samples were stored in a demineralizing solution (lactic acid, pH of 4.4, at 37°C) for 3 days in an incubator. The solution was changed every 4 hours. No changes were performed during their overnight storage. After 3 days, the samples were rinsed with saline solution.
Finally, all samples were embedded in self-cure acrylic resin (Acropars, Marlic, Iran) and each sample was cut in half perpendicular to the tooth surface and parallel to the cementoenamel junction. The samples were then polished with 400-, 600-, and 1200-grit Al2O3 polishing papers (Sof-Lex,3M ESPE, USA), and rinsed with saline solution (Saline wash; Novin Teb Market, Iran).
Microhardness evaluation:
The microhardness of the samples was measured using a Vickers microhardness tester (50 g load for 10 seconds) [16]. In each sample, the microhardness was measured in dentin at 50, 100, and 150 µm distances from the restoration margin, and the mean of the three measurements was calculated as the microhardness of the respective sample. Then, the microhardness measurement was performed for other 3 points in the dentin covered with varnish. The mean of these three measurements was used as the reference dentin value for each sample. The Vickers hardness number (VHN) was calculated using the following formula in which VHN is the Vickers hardness number, F is the applied force, and d is the mean length of the indentation diagonals [17].
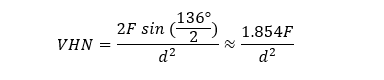
Finally, the mineral index was calculated using the following formula:

Statistical analysis
The data distribution was normal, and data were analyzed using SPSS version 24 with one-way ANOVA followed by the Tukey’s post-hoc test. The level of significance was set at 0.05.
Results
The highest VHN was observed in the ACTIVA+bonding agent (49.46±4.15), followed by Z250 (44.57±3.87), and ACTIVA (43.44±3.76) group (Table 1).
According to the results of one-way ANOVA, there was a significant difference among the groups regarding the mineral index (P<0.001). Pairwise comparisons by the Tukey’s test showed that the mineral index was significantly higher in the ACTIVA+ bonding agent group compared to the Z250 and ACTIVA groups (P<0.05). However, no significant difference was observed between the ACTIVA and Z250 groups in this regard (P>0.05, Figure 1).
Table 1. Measures of central dispersion for the VHN and mineral content index of the study groups
Figure 1. Comparative assessment of VHN and mineral index of the three groups
Discussion
In the recent years, the demand for composite resin materials has been increasing. Despite the fact that composite restorations satisfy patients' esthetic demands, secondary caries, microleakage, crack formation, marginal discoloration, and polymerization shrinkage are among the shortcoming of these restorations [6]. To overcome the shortcomings of composite restorations, bioactive composite resins were introduced to dental market and have attracted the researchers' attention due to their remineralization potential. ACTIVA-BioActive composite resin is one of the newly introduced bioactive composite resins. It releases remineralizing ions such as fluoride, calcium, and phosphate which enhance remineralization.
In the present study, the microhardness of the dentin adjacent to the conventional composite, ACTIVA composite without bonding agent, and ACTIVA composite with bonding agent was evaluated. The samples that received ACTIVA following the bonding agent application showed significantly higher mineral index and thus, the part of null hypothesis regarding the hardness was rejected. However, the microhardness and mineral index were not significantly different between the samples restored with conventional composite with bonding agent and those restored with ACTIVA without a bonding agent.
Different conditioning methods and different acids have been used to condition the dentin surface. EDTA, citric acid, poly acrylic acid, and tannic acid are some of the acids used to etch the dentin surface before the application of resin modified glass ionomer [18,19]. Dentin conditioning improves mechanical bonding by increasing the surface porosities and thus, increases the surface area contacting the restorative material [20]. Moreover, it is believed that acid removes the smear layer and enhances the penetration of bonding agent into dentin. The improved bond strength of resin modified glass ionomer to dentin following acid etching and adhesive application has been reported in the literature [21]. Since ACTIVA composite resin is a material combining the properties of composite resins and resin-modified glass ionomers, the acid etch and bonding agent application improve its bonding properties to dentin.
According to a study by Valanezhad et al. [22], physical characteristics of materials such as surface hardness improve following an increase in bioactive glass particles. Additionally, the authors speculate that an improved chemical bonding between ACTIVA and bonding agent might contribute to increased microhardness and mineral index because of better ion exchange between the ACTIVA and dentin.
The ability of ACTIVA to release mineralizing ions has been shown in some previous studies. According to Porenczuk et al. [11] ACTIVA showed the highest fluoride release during the first day of application (15.552 ppm). The released fluoride decreased the effect of demineralizing solution and enhanced remineralization, and thus, the microhardness level and mineral index increased in ACTIVA samples compared to the samples restored with conventional composite. Various factors such as filler particle size, content and distribution, and also material matrix influence surface hardness [23,24]. Moreover, bioglass fillers present in ACTIVA can improve the pH at the material-tooth interface and decrease demineralization following immersion in a demineralizing solution. Formation of a calcium-phosphate layer at the material-tooth interface after restoring the teeth with composite containing bioactive glass has been shown in a previous study [7]. Due to the presence of bioactive glass particles in ACTIVA composition [11], we expect the formation of calcium-phosphate layer at the ACTIVA-tooth interface. However, we did not investigate this issue, and it should be evaluated in further studies.
Conclusion
Background and Aim: This study aimed to compare the dentin demineralization inhibitory potential of a bioactive composite resin (ACTIVA-BioActive) with a conventional composite resin.
Materials and Methods: In this in vitro experimental study, 45 class V cavities were prepared on the root surface of extracted human third molars. The teeth were then immersed in a demineralizing solution (lactic acid, pH=4.5) at 37˚C for 3 days to induce the formation of secondary caries. The cavities were restored with Single bond 2 + Z250 (group A), ACTIVA BioActive (group B), and Single bond 2 + ACTIVA BioActive (group C). The dentin microhardness was measured close to the restoration margin (marginally exposed dentin), and at approximately 4 mm distance from the margin in a varnish-covered dentin area (protected dentin). Three measurements were made at each site at 50, 100, and 150 µm depths from the external dentin surface. Data were analyzed with one-way ANOVA and Tukey test (alpha=0.05).
Results: The highest Vickers hardness number (VHN) was observed in ACTIVA+ bonding agent (49.46±4.15), followed by Z250 (44.57±3.87), and ACTIVA (43.44±3.76) group. The mineral index was significantly higher in ACTIVA+ bonding agent (76.87±3.80) compared to other groups while no significant difference was observed between ACTIVA (70.85±4.06) and Z250 (71.98±3.09) in this regard (P<0.001).
Conclusion: The results showed that flowable ACTIVA BioActive composite used with Single bond 2 resulted in a significantly smaller reduction in dentin microhardness and reduced secondary caries formation. ACTIVA BioActive with no adhesive had no advantage over Z250 composite.
Keywords: Activa Bioactive Base Liner; Activa Bioactive Restorative; Flowable Hybrid Composite; Tooth Remineralization
Introduction
Dental caries is one of the most common infectious diseases around the world [1]. In recent years, resin restorative materials have been widely used due to their optimal esthetic properties and easy application in the clinical setting [2,3]. Despite many advances in composite resin formulations to improve physical properties, many composite resin materials still contain Bis-GMA, which is responsible for polymerization shrinkage during the setting reaction [4,5]. This shrinkage causes stress, which subsequently results in gap formation, enamel microcracks, microleakage, secondary caries, tooth hypersensitivity, and discoloration around the restoration margins [6]. This highlights the need for developing effective antibacterial and bioactive restorative materials that can prevent bacterial colonization and secondary caries [7], subsequently increasing the longevity of composite restorations [8].
In recent years, a new generation of restorative materials known as bioactive composite resins was introduced to dental market. These materials can form chemical bonds to tooth structure without adhesive agents. Absence of monomers responsible for polymerization shrinkage in the composition of bioactive composite resins prevents further shrinkage and stress during polymerization process. Additionally, silver bioglass fillers and amorphous phosphopeptide calcium phosphate present in their composition act as a scaffold or matrix for further remineralization process [9,10]. In a study conducted by Chatzistavrou et al. [7], a composite resin containing silver bioglass enhanced the remineralization properties by increasing the formation of calcium-phosphate apatite-like phase in field emission scanning electron microscopy analysis after 14 days. In addition, these materials have both self-cure and light-cure setting modes, which decrease the concerns regarding the curing depth and microleakage [9,10].
ACTIVA-BioActive is one of the newly introduced bioactive materials. In fact, ACTIVA- BioActive is a hydrophilic resin-modified glass ionomer cement enriched with bioglass and fortified with a patented rubberized polymer resin [11]. The ion release, microleakage, and bond strength of ACTIVA composite resin have been previously evaluated with reportedly promising results. Porenczuk et al. [11] reported fluoride release from ACTIVA composite, which was the highest in the first 24 hours. Zmener et al. [12] reported significantly higher flexural strength and flexural fatigue of ACTIVA composite compared to conventional glass ionomer cement and flowable composite resin. However, the authors did not find any study evaluating the effect of ACTIVA-BioActive composite resin as a bioactive material on microhardness of the adjacent dentin. Thus, due to the limited information regarding the effect of this material on the hardness of adjacent dentin and its remineralization potential, the present study aimed to compare the microhardness of dentin adjacent to ACTIVA composite with and without a bonding agent, and Z250 composite resin. The null hypothesis was that the microhardness values of dentin adjacent to ACTIVA with and without a bonding agent and Z-250 composite would not be significantly different.
Materials and Methods
This in vitro experimental study was approved by the ethics committee of Tehran University of Medical Sciences (IR.TUMS.DENTISTRY. REC.1398.122).
Sample size:
The minimum sample size was calculated to be 15 specimens in each experimental group using one-way ANOVA feature of PASS 11 (NCSS, LLC., Kaysville, Utah, USA), considering alpha=0.05, beta=0.2, mean standard deviation of microhardness equal to 4.97, and effect size of 0.49 [13].
Sample preparation:
Forty-five freshly extracted human third molars were used in the present study. The teeth had no caries, physical anomaly, or crack, and were stored in saline solution at 4°C until use. After removing the residual soft tissue and calculus, the teeth were stored in 0.5% chloramine T (Wako Pure Chemical Industry, Osaka, Japan) for one week for disinfection. To remove cement layer from the root surface, the samples were polished with 1000-grit silicon carbide paper (Sof-Lex, 3M ESPE, USA) under water irrigation.
Each sample was split in half in mesiodistal direction to obtain two symmetrical halves. The samples were then randomly divided into 3 groups (n=15). Standard class V cavities (2.5 mm in diameter and 1 mm in depth) were prepared on the samples 1 mm below the cementoenamel junction using a fine diamond bur (Intensiv #2201, INTENSIV SA, Switzerland) in a high-speed handpiece under water coolant [14]. The bur used in the handpiece was changed after each 5 preparations. Afterwards, the prepared cavities were cleaned using pumice slurry. Then, each sample was restored according to its group as follows:
Z-250: The samples were etched with 37% phosphoric acid (Scotchbond, 3M ESPE, USA) for 15 seconds, followed by 10 seconds of rinsing with water. Then, a cotton pellet was used to remove excess water from each cavity. The bonding agent (Single bond, 3M ESPE, USA) was applied in each cavity with a microbrush and was dried with gentle air flow for 10 seconds. The second layer of bonding agent was applied with the same approach. The samples were cured using a polywave LED curing unit (Woodpecker LED Curing, Guilin Woodpecker Medical Instrument Co., Guilin, China) with 1000 mW/cm2 power intensity for 20 seconds. Next, one layer of composite resin (Z250; 3M ESPE, MN, USA) was placed in the cavity and light-cured for 40 seconds.
ACTIVA: No etching or adhesive agent was used. Flowable ACTIVA™ BioActive composite resin (Pulpdent, Watertown, MA, USA) was placed in the cavities and light-cured for 20 seconds.
ACTIVA+ bonding agent: The samples were etched, and the bonding agent was used in the same manner as explained in group A. Then, ACTIVA™ BioActive flowable composite was placed in the cavities and light-cured for 20 seconds.
Finally, the cavity margins were polished using 600-, 800-, 1000-, and 1200-grit polishing discs (3M, St. Paul, MN, USA) under water spray and rinsed with deionized water. All surfaces of the samples except the composite surface and 1 mm dentin adjacent to the composite were covered with 2 layers of acid-resistant varnish. The samples were then subjected to thermocycling in water baths between 5°C and 55°C with a dwell time of 15 seconds for 10,000 cycles corresponding to one year of clinical performance [15].
Demineralization process:
The samples were stored in a demineralizing solution (lactic acid, pH of 4.4, at 37°C) for 3 days in an incubator. The solution was changed every 4 hours. No changes were performed during their overnight storage. After 3 days, the samples were rinsed with saline solution.
Finally, all samples were embedded in self-cure acrylic resin (Acropars, Marlic, Iran) and each sample was cut in half perpendicular to the tooth surface and parallel to the cementoenamel junction. The samples were then polished with 400-, 600-, and 1200-grit Al2O3 polishing papers (Sof-Lex,3M ESPE, USA), and rinsed with saline solution (Saline wash; Novin Teb Market, Iran).
Microhardness evaluation:
The microhardness of the samples was measured using a Vickers microhardness tester (50 g load for 10 seconds) [16]. In each sample, the microhardness was measured in dentin at 50, 100, and 150 µm distances from the restoration margin, and the mean of the three measurements was calculated as the microhardness of the respective sample. Then, the microhardness measurement was performed for other 3 points in the dentin covered with varnish. The mean of these three measurements was used as the reference dentin value for each sample. The Vickers hardness number (VHN) was calculated using the following formula in which VHN is the Vickers hardness number, F is the applied force, and d is the mean length of the indentation diagonals [17].
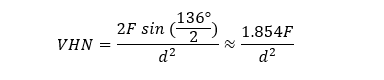
Finally, the mineral index was calculated using the following formula:

Statistical analysis
The data distribution was normal, and data were analyzed using SPSS version 24 with one-way ANOVA followed by the Tukey’s post-hoc test. The level of significance was set at 0.05.
Results
The highest VHN was observed in the ACTIVA+bonding agent (49.46±4.15), followed by Z250 (44.57±3.87), and ACTIVA (43.44±3.76) group (Table 1).
According to the results of one-way ANOVA, there was a significant difference among the groups regarding the mineral index (P<0.001). Pairwise comparisons by the Tukey’s test showed that the mineral index was significantly higher in the ACTIVA+ bonding agent group compared to the Z250 and ACTIVA groups (P<0.05). However, no significant difference was observed between the ACTIVA and Z250 groups in this regard (P>0.05, Figure 1).
Table 1. Measures of central dispersion for the VHN and mineral content index of the study groups
Figure 1. Comparative assessment of VHN and mineral index of the three groups
Discussion
In the recent years, the demand for composite resin materials has been increasing. Despite the fact that composite restorations satisfy patients' esthetic demands, secondary caries, microleakage, crack formation, marginal discoloration, and polymerization shrinkage are among the shortcoming of these restorations [6]. To overcome the shortcomings of composite restorations, bioactive composite resins were introduced to dental market and have attracted the researchers' attention due to their remineralization potential. ACTIVA-BioActive composite resin is one of the newly introduced bioactive composite resins. It releases remineralizing ions such as fluoride, calcium, and phosphate which enhance remineralization.
In the present study, the microhardness of the dentin adjacent to the conventional composite, ACTIVA composite without bonding agent, and ACTIVA composite with bonding agent was evaluated. The samples that received ACTIVA following the bonding agent application showed significantly higher mineral index and thus, the part of null hypothesis regarding the hardness was rejected. However, the microhardness and mineral index were not significantly different between the samples restored with conventional composite with bonding agent and those restored with ACTIVA without a bonding agent.
Different conditioning methods and different acids have been used to condition the dentin surface. EDTA, citric acid, poly acrylic acid, and tannic acid are some of the acids used to etch the dentin surface before the application of resin modified glass ionomer [18,19]. Dentin conditioning improves mechanical bonding by increasing the surface porosities and thus, increases the surface area contacting the restorative material [20]. Moreover, it is believed that acid removes the smear layer and enhances the penetration of bonding agent into dentin. The improved bond strength of resin modified glass ionomer to dentin following acid etching and adhesive application has been reported in the literature [21]. Since ACTIVA composite resin is a material combining the properties of composite resins and resin-modified glass ionomers, the acid etch and bonding agent application improve its bonding properties to dentin.
According to a study by Valanezhad et al. [22], physical characteristics of materials such as surface hardness improve following an increase in bioactive glass particles. Additionally, the authors speculate that an improved chemical bonding between ACTIVA and bonding agent might contribute to increased microhardness and mineral index because of better ion exchange between the ACTIVA and dentin.
The ability of ACTIVA to release mineralizing ions has been shown in some previous studies. According to Porenczuk et al. [11] ACTIVA showed the highest fluoride release during the first day of application (15.552 ppm). The released fluoride decreased the effect of demineralizing solution and enhanced remineralization, and thus, the microhardness level and mineral index increased in ACTIVA samples compared to the samples restored with conventional composite. Various factors such as filler particle size, content and distribution, and also material matrix influence surface hardness [23,24]. Moreover, bioglass fillers present in ACTIVA can improve the pH at the material-tooth interface and decrease demineralization following immersion in a demineralizing solution. Formation of a calcium-phosphate layer at the material-tooth interface after restoring the teeth with composite containing bioactive glass has been shown in a previous study [7]. Due to the presence of bioactive glass particles in ACTIVA composition [11], we expect the formation of calcium-phosphate layer at the ACTIVA-tooth interface. However, we did not investigate this issue, and it should be evaluated in further studies.
Conclusion
Within the limitations of this study, the following results were obtained:
- Flowable ACTIVA BioActive composite significantly limited the reduction of dentin microhardness and decreased the formation of secondary caries when used together with Single bond 2.
- ACTIVA BioActive composite with no bonding agent had no superiority over the conventional composite resin.
Type of Study: Original article |
Subject:
Restorative Dentistry
References
1. Craig RG, Powers JM. Craig's Restorative Dental Materials. 14th ed. St. Louis: Mosby; 2018. Chapter 1, Biological considerations; p. 1-15.
2. Cheng L, Zhang K, Zhang N, Melo MAS, Weir MD, Zhou XD, Bai YX, Reynolds MA, Xu HHK. Developing a New Generation of Antimicrobial and Bioactive Dental Resins. J Dent Res. 2017 Jul;96(8):855-63. [DOI:10.1177/0022034517709739] [PMID] []
3. Savarino L, Breschi L, Tedaldi M, Ciapetti G, Tarabusi C, Greco M, Giunti A, Prati C. Ability of restorative and fluoride releasing materials to prevent marginal dentine demineralization. Biomaterials. 2004 Mar;25(6):1011-7. [DOI:10.1016/S0142-9612(03)00628-8] [PMID]
4. Brunthaler A, König F, Lucas T, Sperr W, Schedle A. Longevity of direct resin composite restorations in posterior teeth. Clin Oral Investig. 2003 Jun;7(2):63-70. [DOI:10.1007/s00784-003-0206-7] [PMID]
5. Opdam NJ, Loomans BA, Roeters FJ, Bronkhorst EM. Five-year clinical performance of posterior resin composite restorations placed by dental students. J Dent. 2004 Jul;32(5):379-83. [DOI:10.1016/j.jdent.2004.02.005] [PMID]
6. M R, Sajjan GS, B N K, Mittal N. Effect of different placement techniques on marginal microleakage of deep class-II cavities restored with two composite resin formulations. J Conserv Dent. 2010 Jan;13(1):9-15. [DOI:10.4103/0972-0707.62633] [PMID] []
7. Chatzistavrou X, Lefkelidou A, Papadopoulou L, Pavlidou E, Paraskevopoulos KM, Fenno JC, Flannagan S, González-Cabezas C, Kotsanos N, Papagerakis P. Bactericidal and Bioactive Dental Composites. Front Physiol. 2018 Feb 16;9:103. [DOI:10.3389/fphys.2018.00103] [PMID] []
8. Chatzistavrou X, Fenno JC, Faulk D, Badylak S, Kasuga T, Boccaccini AR, et al. Fabrication and characterization of bioactive and antibacterial composites for dental applications. Acta Biomater. 2014 Aug;10(8):3723-32. [DOI:10.1016/j.actbio.2014.04.030] [PMID]
9. Alkhudhairy FI, Ahmad ZH. Comparison of Shear Bond Strength and Microleakage of Various Bulk-fill Bioactive Dentin substitutes: An in vitro Study. J Contemp Dent Pract. 2016 Dec 1;17(12):997-1002. [DOI:10.5005/jp-journals-10024-1970] [PMID]
10. Croll TP, Berg JH, Donly KJ. Dental repair material: a resin-modified glass-ionomer bioactive ionic resin-based composite. Compend Contin Educ Dent. 2015 Jan;36(1):60-5.
11. Porenczuk A, Jankiewicz B, Naurecka M, Bartosewicz B, Sierakowski B, Gozdowski D, et al. A comparison of the remineralizing potential of dental restorative materials by analyzing their fluoride release profiles. Adv Clin Exp Med. 2019 Jun;28(6):815-23. [DOI:10.17219/acem/94140] [PMID]
12. Zmener O, Pameijer CH, Hernández S. Resistance against bacterial leakage of four luting agents used for cementation of complete cast crowns. Am J Dent. 2014 Feb;27(1):51-5.
13. Khamverdi Z, Kordestani M, Soltanian AR. Effect of Proanthocyanidin, Fluoride and Casein Phosphopeptide Amorphous Calcium Phosphate Remineralizing Agents on Microhardness of Demineralized Dentin. J Dent (Tehran). 2017 Mar;14(2):76-83.
14. Elsayed HE, Hammouda HE. Comparative Evaluation of Microleakage of Different Class V Cavity Preparation Restored with Composite in Primary Molars: An In Vitro Study. Mansoura Journal of Dentistry. 2023;10(1):48-52. [DOI:10.21608/mjd.2023.288116]
15. Gale MS, Darvell BW. Thermal cycling procedures for laboratory testing of dental restorations. J Dent. 1999 Feb;27(2):89-99. [DOI:10.1016/S0300-5712(98)00037-2] [PMID]
16. Franc J, Moravec P, Dědič V, Roy U, Elhadidy H, Minárik P, et al. Microhardness study of Cd1-x ZnxTe1-ySey crystals for X-ray and gamma ray detectors. Materials Today Communications. 2020 Sep 1;24:101014. [DOI:10.1016/j.mtcomm.2020.101014]
17. Moreira FD, Kleinberg MN, Arruda HF, Freitas FN, Parente MM, De Albuquerque VH, et al. A novel Vickers hardness measurement technique based on Adaptive Balloon Active Contour Method. Expert systems with applications. 2016 Mar 1;45:294-306. [DOI:10.1016/j.eswa.2015.09.025]
18. Imbery TA, Namboodiri A, Duncan A, Amos R, Best AM, Moon PC. Evaluating dentin surface treatments for resin-modified glass ionomer restorative materials. Oper Dent. 2013 Jul-Aug;38(4):429-38. [DOI:10.2341/12-162-L] [PMID]
19. Yamamoto K, Kojima H, Tsutsumi T, Oguchi H. Effects of tooth-conditioning agents on bond strength of a resin-modified glass-ionomer sealant to enamel. J Dent. 2003 Jan;31(1):13-8. [DOI:10.1016/S0300-5712(02)00086-6] [PMID]
20. Poggio C, Beltrami R, Scribante A, Colombo M, Lombardini M. Effects of dentin surface treatments on shear bond strength of glass-ionomer cements. Ann Stomatol (Roma). 2014 Mar 31;5(1):15-22. [DOI:10.11138/ads/2014.5.1.015]
21. Imbery TA, Namboodiri A, Duncan A, Amos R, Best AM, Moon PC. Evaluating dentin surface treatments for resin-modified glass ionomer restorative materials. Oper Dent. 2013 Jul-Aug;38(4):429-38. [DOI:10.2341/12-162-L] [PMID]
22. Valanezhad A, Odatsu T, Udoh K, Shiraishi T, Sawase T, Watanabe I. Modification of resin modified glass ionomer cement by addition of bioactive glass nanoparticles. J Mater Sci Mater Med. 2016 Jan;27(1):3. [DOI:10.1007/s10856-015-5614-0] [PMID]
23. Kasraei S, Haghi S, Farzad A, Malek M, Nejadkarimi S. Comparative of flexural strength, hardness, and fluoride release of two bioactive restorative materials with RMGI and composite resin. Brazilian Journal of Oral Sciences. 2022 May 9;21:e225263. [DOI:10.20396/bjos.v21i00.8665263]
24. Garcia IM, Balhaddad AA, Aljuboori N, Ibrahim MS, Mokeem L, Ogubunka A, Collares FM, de Melo MAS. Wear Behavior and Surface Quality of Dental Bioactive Ions-Releasing Resins Under Simulated Chewing Conditions. Front Oral Health. 2021 Feb 12;2:628026. [DOI:10.3389/froh.2021.628026] [PMID] []
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



