Volume 9, Issue 3 (9-2024)
J Res Dent Maxillofac Sci 2024, 9(3): 151-158 |
Back to browse issues page
Ethics code: IR.SSU.REC.1399.314
Clinical trials code: IRCT20210306050606N1
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Barzegar M, Ghadirian H, Roustaeizade Shooroki Z, Yektaie M A, Pouyafard A. Comparative Efficacy of Triadent and Ora-Aid Mucoadhesive Patch for Treatment of Minor Aphthous Ulcers: A Clinical Trial. J Res Dent Maxillofac Sci 2024; 9 (3) :151-158
URL: http://jrdms.dentaliau.ac.ir/article-1-572-en.html
URL: http://jrdms.dentaliau.ac.ir/article-1-572-en.html
Mohsen Barzegar1 

 , Hamidreza Ghadirian2
, Hamidreza Ghadirian2 

 , Zahra Roustaeizade Shooroki3
, Zahra Roustaeizade Shooroki3 

 , Mohammad Amin Yektaie4
, Mohammad Amin Yektaie4 

 , Adele Pouyafard *5
, Adele Pouyafard *5 




 , Hamidreza Ghadirian2
, Hamidreza Ghadirian2 

 , Zahra Roustaeizade Shooroki3
, Zahra Roustaeizade Shooroki3 

 , Mohammad Amin Yektaie4
, Mohammad Amin Yektaie4 

 , Adele Pouyafard *5
, Adele Pouyafard *5 


1- Department of Oral and Maxillofacial Surgery, School of Dentistry, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
2- School of Dentistry, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Department of Oral and Maxillofacial Medicine, School of Dentistry, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Department of Oral and Maxillofacial Surgery, School of Dentistry, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Department of Oral and Maxillofacial Medicine, School of Dentistry, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. ,a.pouyafard@gmail.com
2- School of Dentistry, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
3- Department of Oral and Maxillofacial Medicine, School of Dentistry, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4- Department of Oral and Maxillofacial Surgery, School of Dentistry, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
5- Department of Oral and Maxillofacial Medicine, School of Dentistry, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. ,
Full-Text [PDF 362 kb]
(477 Downloads)
| Abstract (HTML) (1299 Views)
Table 3. Comparison of the mean reduction in diameter of ulcers at different time points between the two groups
Table 4. Comparison of the mean reduction in pain and burning VAS score at different time points between the two groups
Discussion
Mucoadhesive patches have been introduced as a novel controlled targeted drug delivery system in the field of dentistry [12]. This study compared the effects of Triadent and Ora-Aid mucoadhesive patch on pain severity, burning sensation, and size of minor aphthous ulcers. The present study is among the very first to assess the efficacy of Ora-Aid mucosal adhesive in Iran. Ora-Aid (TBM, Gwangju, Korea) is a mixture of polymers and vitamin E. It is a non-eugenol protective dressing composed of hydrophilic high-density polymers including hydroxy ethyl cellulose according to the latest pharmaceutical standards of South Korea and the United States Pharmacopeia, encapsulated in water-insoluble mucoadhesive synthetic cellulose. It also contains vitamin E which has wound healing and homeostatic effects. The adhesive surface of Ora-Aid is placed directly on the oral mucosa, inducing the oral mucosa to form a protective layer. It aids effective hemostasis, and provides physical protection against foods, bacterial irritants, and cigarette smoke [13]. It is a possible alternative to topical corticosteroids such as triamcinolone acetonide.
Several factors may be used for assessment of treatment efficacy such as reduction in pain and burning sensation, reduction of ulcer size, reduction of number of inflammatory cells in microscopic assessment, and reduction of the number of ulcers [14-16]. In the present study, pain and burning sensation and size of ulcers were evaluated. Pain and burning sensation were evaluated by using a VAS, which is highly reliable for acute and chronic pains and oral symptoms such as ulcers [9, 10].
Diameter of the largest ulcer was measured in the present study, which is a highly accurate and frequently used technique [10, 17]. However, Deshmukh and Bagewadi [18] used a sterile Vernier caliper for measurement of ulcer diameter which is less accurate than the present technique. Since the recovery period of RAS is 7 to 10 days, the treatment course was considered to be 7 days in the present study similar to many previous studies [17, 18]. Fani et al. [7] only evaluated the parameters on day 7, which is less accurate compared with the present methodology [19]. Different medication forms may be used for treatment of ulcers including syrup, creme, mucoadhesive paste, and mouthwash [20]. In the present study, triamcinolone mucoadhesive paste was used similar to studies by Fani et al. [7], Ahadian et al. [17], Mehdipour et al. [21], and Babadi and Poodeh [10], and was compared with Ora-Aid mucoadhesive patch, which is a novel treatment strategy. Unlike Triadnet, which does not have a pleasant taste, Ora-Aid mucoadhesive patch does not have an unpleasant taste and is better accepted by patients. Fani et al. [7], Mehdipour et al. [21], and Babadi and Poodeh [10] used Triadent in comparison with phenytoin mouthwash, zinc-containing mouthwash, and Salvizan gel, which were different from the methodology of the present study. The nature of the two treatments was not the same in the abovementioned studies; thus, an accurate comparison could not be made. However, in the present study, two mucoadhesive substances were compared in different forms, which is a strength, and enables more accurate comparison of their efficacy, similar to a study by Ahadian et al. [17].
In the current study, Ora-Aid was more effective in reduction of pain and burning sensation while Triadent was more effective in reduction of size of ulcers. Babadi and Poodeh [10] reported that Triadent effectively decreased the diameter of ulcers, which was in agreement with the present results. Trident has a paste-like consistency and cannot completely cover the wound; thus, it is partially rinsed off by the saliva. This statement may explain why Ora-Aid was more effective in reduction of pain and burning sensation. This result was similar to the findings of Rodrigues et al. [13] who reported that Ora-Aid mucoadhesive patch was effective for postoperative pain reduction after gingival grafting. Trident is a moderate to strong corticosteroid and because of its strong anti-inflammatory properties, it is expected to be more effective in reduction of size of ulcers.
To the best of the authors’ knowledge, this study is the first to examine the efficacy of Ora-Aid mucoadhesive patches for aphthous ulcers. Therefore, lack of similar studies due to the novelty of the topic is one of the main limitations of the present study. Another limitation of this study was difficult patient recruitment due to the COVID-19 pandemic. In addition, inter-individual differences in healing power and immune system capacity can serve as a confounder and could not be controlled for in the present study, which was another limitation. Future studies with a larger sample size are required to achieve more reliable results.
Conclusion
Both Triadent and Ora-Aid significantly decreased pain and burning sensation and size of minor aphthous lesions. However, Triadent was significantly more effective for reduction of size of lesions while Ora-Aid was significantly more effective for reduction of pain and burning sensation. Thus, Ora-Aid may be used as an alternative to Triadent for treatment of RAS.
Acknowledgement
The authors would like to thank the Vice-Chancellor for Technology and Research of Shahid Sadoughi University of Medical Sciences in Yazd for approving and supporting this research project (Grant no. 1116).
Full-Text: (528 Views)
Abstract
Background and Aim: This study aimed to compare the effects of Triadent (triamcinolone mucoadhesive paste) and Ora-Aid mucoadhesive patch on the severity of pain, burning sensation, and size of minor aphthous ulcers.
Materials and Methods: This double-blind clinical trial was conducted on 40 patients with minor aphthous ulcers. The patients were randomly assigned to two groups (n=20) to use Triadent paste or Ora-Aid mucoadhesive patch (3 times/day). The severity of pain and burning sensation of patients was quantified by a visual analog scale (VAS). Size of lesions was also measured before, and at 3, 5, and 7 days after the treatment by using a graded paper placed next to the lesions. Data were analyzed by the t-test and the Mann-Whitney test (alpha=0.05).
Results: The mean reduction in lesion diameter at 3 days (compared with baseline) was significantly greater in the Ora-Aid than the Triadent group (P=0.000). However, the mean reduction in lesion diameter at 5 and 7 days was significantly greater in the Triadent group (P=0.000). The mean reduction in the severity of pain and burning sensation was significantly greater in the Ora-Aid group than the Triadent group at 3, 5, and 7 days (P=0.000).
Conclusion: Both Triadent and Ora-Aid significantly decreased pain, burning sensation, and size of minor aphthous lesions. However, Triadent was significantly more effective for reduction of size of lesions while Ora-Aid was significantly more effective for reduction of pain and burning sensation.
Keywords: Stomatitis, Aphthous; Triamcinolone Acetonide, Humans
Introduction
Recurrent aphthous stomatitis (RAS) is among the most common diseases of the oral mucosa. The prevalence of RAS ranges from 5% to 25% [1]. The prevalence of RAS in the Iranian population is estimated at 20%-26% [2]. Evidence shows that RAS has a higher prevalence in females than males. It is a multifactorial disease; however, its exact etiology has yet to be clearly understood. Genetics, malnutrition, allergy to some medications, stress, local trauma, hormonal imbalance, infection, poor oral hygiene, and underlying conditions such as iron deficiency anemia, folic acid deficiency, impaired absorption of vitamin B12, cyclic neutropenia, and Celiac disease have been proposed as influential factors on development of RAS. RAS lesions are painful and manifest as round or oval-shaped lesions with a distinct border that are covered with a white-gray pseudo-membrane. They also have an erythematous margin. RAS affects non-keratinized mucosa especially the lip mucosa, buccal mucosa, borders and ventral surface of the tongue, and floor of the mouth. The lesions often heal within 10 to 14 days with no scarring. There is no definite paraclinical test for the diagnosis of RAS [3, 4]. Thus, RAS is often diagnosed according to patient history and clinical manifestations. Taking a precise medical history can rule out the Crohn’s disease, Celiac disease, cyclic neutropenia, HIV infection, and Behcet’s syndrome.
Currently, the treatment of RAS is non-specific and empirical in most cases, and none of the current treatments can lead to permanent recovery. Treatment of recurrent aphthous ulcers is performed aiming to alleviate the symptoms, accelerate the healing process, and decrease the frequency of recurrence [5]. Treatment should be promptly started in the first hours following development of lesions. Identification and elimination of predisposing factors may decrease the frequency of recurrence. Analgesics, local anesthetic agents, anti-inflammatory drugs, anti-septic drugs, steroids, sucralfate, tetracycline, and silver nitrate may be prescribed for local and topical application [6].
Immune system regulators such as dapsone, thalidomide, cyclosporine A, interferon alpha, colchicine, prednisolone, pentoxifylline, methotrexate, tumor necrosis factor antagonists, and azathioprine may also be effective in patients with resistant forms of RAS, or in case of RAS accompanied by a systemic condition. In general, RAS lesions have a favorable prognosis, and often heal spontaneously within a couple of days [6].
Triamcinolone acetonide is a moderate to strong corticosteroid which is derived from prednisolone fluorinate. Its mucoadhesive paste is used as an adjunct for temporary alleviation of symptoms in oral and gingival inflammatory conditions such as RAS [7]. Its mouthwash and crème forms may be used for oral mucosal lesions. Its mouthwash, however, has a short contact time with the lesions, compared with other forms of medication. Crèmes can be easily applied; however, they are also easily washed-out during speech, mastication, and tongue movements. Addition of a mucoadhesive polymer to its crème formulation can prolong its contact time. Thus, triamcinolone mucoadhesive paste was developed for prolonged adhesion. Triadent is a triamcinolone mucoadhesive paste (0.1%) available in the Iranian market [7].
It should be noted that high substantivity of mucoadhesive materials increases the risk of systemic absorption of corticosteroids, which can lead to complications following frequent long-term use [8]. Considering the systemic side effects and complications of steroids such as suppression of the hypothalamus-pituitary-adrenal axis, epithelial atrophy, hyper-pigmentation, candidiasis, and acne, this study aimed to compare the effects of Triadent and Ora-Aid mucoadhesive patch for treatment of minor aphthous ulcers.
Materials and Methods
This clinical trial was conducted at the Oral Medicine Department of School of Dentistry, Yazd University of Medical Sciences between 2020 and 2021. The study protocol was approved by the ethics committee of the university (IR.SSU.REC.1399.314), and registered in the Iranian Registry of Clinical Trials (IRCT20210306050606N1).
Trial design:
A randomized single-blind clinical trial was conducted in which one group received Triadent triamcinolone mucoadhesive paste and the other group received Ora-Aid mucoadhesive patch for treatment of RAS ulcers. The results were reported in accordance with the Consolidated Standards of Reporting Trials.
Participants, eligibility criteria, and settings:
The inclusion criteria were age between 20-40 years, no more than 1 day had to be passed since the development of minor aphthous ulcers, no history of using any other treatment for the aphthous ulcer, no systemic disease related to development of RAS, absence of ulcers on the tonsils and posterior third of the oral cavity, and willingness for participation in the study and signing informed consent forms.
It is worth mentioning that patients were included in this study who had no suspected sign/symptom of any systemic condition related to RAS according to the requested necessary laboratory tests and based on a detailed medical history and clinical examination, and were diagnosed with idiopathic RAS.
The exclusion criteria were consumption of another medication simultaneous with the treatment of RAS, no or irregular use of the prescribed medication as self-reported by patient, not showing up for the recall session, and symptoms of Behcet’s disease (RAS ulcers, recurrent genital ulcers, cutaneous lesions, and ocular lesions).
The sample consisted of 40 eligible patients with RAS presenting to the Oral Medicine Department of Yazd Shahid Sadoughi University of Medical Sciences in 2020-2021.
Interventions:
After comprehensive clinical examination and taking a precise medical history, eligible patients with a confirmed diagnosis of RAS were enrolled. After obtaining written informed consent from the patients, their age, gender, size of lesion, and severity of pain and burning sensation were recorded in a predesigned checklist. The size of lesions was recorded as the largest diameter of the wound. For this purpose, 1 mm grade paper was placed next to the lesions (Figure 1). The level of pain and burning sensation of lesions was quantified by using a visual analog scale (VAS) [9, 10].The patients were instructed on how to use a 100-mm VAS and record their pain level and burning sensation. Score 0 indicated no pain and score 100 indicated maximum pain imaginable. The patients were randomly assigned to two groups by using a table of random numbers. An assistant assigned either medication A or medication B to each patient using a table of random numbers. The Triadent triamcinolone acetonide (0.1%) mucoadhesive paste (Raha Pharmaceuticals, Iran) was used for 20 patients in group A while Ora-Aid mucoadhesive patch (TBM, South Korea) was used for 20 patients in group B. It should be noted that the medication and written instructions on how to use it were placed in identical boxes with a specific code and were delivered to patients by an assistant. The patients in the Triadent group were instructed to first gently dry the mucosal wound surface and then apply some medication over the aphthous ulcer by an applicator. Next, they had to remain their mouth open for 2 minutes. The patients were instructed to apply the medication 3 times/day after meals and refrain from eating and drinking for up to 1 hour after that. Duration of use of medications was determined according to the manufacturer’s instructions. The patients in the Ora-Aid group were instructed to first rinse the ulcer with saline, and then select the proper size mucoadhesive patch, dry the ulcer with a gauze, and apply the patch over the ulcer and compress it for 5-10 seconds to stick to the wound. The patients were recalled after 3, 5, and 7 days following initiation of treatment [11], and their level of pain and burning sensation was assessed by a VAS. Also, the wound size was measured again as explained earlier. Duration of recovery was also recorded. Patients with several aphthous lesions were instructed to use the medication for all ulcers but the largest and most accessible lesion was included in the study.
Sample size calculation:
The sample size was calculated to be 20 in each of the two groups according to a previous study [12] assuming alpha=5%, and study power of 80% using the following formula:
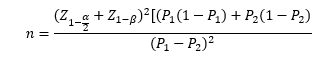
Interim analyses and stopping guidelines:
No interim analyses were performed and no stopping guidelines were established.
Randomization:
The patients were randomly assigned to two groups by using a table of random numbers. An assistant assigned either medication A or medication B to each patient using a table of random numbers. The medications along with their written instructions for use were supplied in similar packages and were randomly delivered to patients.
Blinding:
The assistant and the patients were blinded to their group allocation.
Figure 1. Measuring the largest diameter (length) of a minor aphthous ulcer
Statistical analysis:
The qualitative data were compared between the two groups by the Chi-square test. The quantitative data were compared between the two groups by the t-test. The Mann-Whitney test was applied to compare ordinal variables between the two groups, and repeated measures ANOVA was applied for within-group and between-group comparisons. The Friedman test was also used to analyze the reduction in pain and burning sensation over time. All statistical analyses were carried out using SPSS version 25 (SPSS Inc., IL, USA) at 0.05 level of significance.
Results
The sample consisted of 40 patients including 16 males and 24 females. There were 8 males (40%) and 12 females (60%) in each group. Thus, the two groups had no significant difference in gender distribution as shown by the Chi-square test (P=1.00). The mean age of the patients was 29.40±6.38 years (range 15 to 40 years). The mean age was 30.35±6.07 years in the Triadent group and 28.45±6.70 years in the Ora-Aid group with no significant difference between them as shown by the t-test (P=0.354). Table 1 presents the mean maximum diameter of ulcers (in millimeters) at different time points in the two groups. As shown by the Wilcoxon test, the mean diameter of ulcers was not significantly different between the two groups at 1 and 3 days (P>0.05). However, at 5 (P=0.019) and 7 (P=0.023) days, the mean diameter of the ulcers was significantly smaller in the Ora-Aid group.
Table 1. Mean maximum diameter of ulcers (in millimeters) at different time points in the two groups
Table 2 presents the mean severity of pain and burning sensation (VAS scores). The Mann-Whitney test showed no significant difference in this regard between the two groups at 1, 5, and 7 days (P>0.05). However, the VAS pain and burning score was significantly lower in the Triadent group at 3 days (P=0.015).
Repeated measures ANOVA showed a significant difference between the two groups in the trend of reduction in diameter of ulcers over time (P=0.037, Figure 2).
Table 2. Mean severity of pain and burning sensation (VAS scores) in the two groups
According to the Mann-Whitney test (Table 3), the mean reduction in the diameter of ulcers on day 3 (between days 1 to 3) was significantly greater in the Ora-Aid group than in the Triadent group (P=0.000). However, the mean reduction in diameter of ulcers on day 5 (between 3-5 days) and on day 7 (between 5 to 7 days) was significantly greater in the Triadent group (P=0.000).
The Friedman test showed that the reduction in severity of pain and burning sensation over time was significantly different between the two groups (P=0.022, Figure 3). According to the Mann-Whitney test (Table 4), the mean reduction in pain and burning VAS score was significantly greater in the Ora-Aid group at 3 (between days 1 to 3), 5 (between days 3 to 5), and 7 (between days 5 to 7) days (P=0.000 for all).
Figure 2. Changes in diameter of ulcers over time in the two groups
Background and Aim: This study aimed to compare the effects of Triadent (triamcinolone mucoadhesive paste) and Ora-Aid mucoadhesive patch on the severity of pain, burning sensation, and size of minor aphthous ulcers.
Materials and Methods: This double-blind clinical trial was conducted on 40 patients with minor aphthous ulcers. The patients were randomly assigned to two groups (n=20) to use Triadent paste or Ora-Aid mucoadhesive patch (3 times/day). The severity of pain and burning sensation of patients was quantified by a visual analog scale (VAS). Size of lesions was also measured before, and at 3, 5, and 7 days after the treatment by using a graded paper placed next to the lesions. Data were analyzed by the t-test and the Mann-Whitney test (alpha=0.05).
Results: The mean reduction in lesion diameter at 3 days (compared with baseline) was significantly greater in the Ora-Aid than the Triadent group (P=0.000). However, the mean reduction in lesion diameter at 5 and 7 days was significantly greater in the Triadent group (P=0.000). The mean reduction in the severity of pain and burning sensation was significantly greater in the Ora-Aid group than the Triadent group at 3, 5, and 7 days (P=0.000).
Conclusion: Both Triadent and Ora-Aid significantly decreased pain, burning sensation, and size of minor aphthous lesions. However, Triadent was significantly more effective for reduction of size of lesions while Ora-Aid was significantly more effective for reduction of pain and burning sensation.
Keywords: Stomatitis, Aphthous; Triamcinolone Acetonide, Humans
Introduction
Recurrent aphthous stomatitis (RAS) is among the most common diseases of the oral mucosa. The prevalence of RAS ranges from 5% to 25% [1]. The prevalence of RAS in the Iranian population is estimated at 20%-26% [2]. Evidence shows that RAS has a higher prevalence in females than males. It is a multifactorial disease; however, its exact etiology has yet to be clearly understood. Genetics, malnutrition, allergy to some medications, stress, local trauma, hormonal imbalance, infection, poor oral hygiene, and underlying conditions such as iron deficiency anemia, folic acid deficiency, impaired absorption of vitamin B12, cyclic neutropenia, and Celiac disease have been proposed as influential factors on development of RAS. RAS lesions are painful and manifest as round or oval-shaped lesions with a distinct border that are covered with a white-gray pseudo-membrane. They also have an erythematous margin. RAS affects non-keratinized mucosa especially the lip mucosa, buccal mucosa, borders and ventral surface of the tongue, and floor of the mouth. The lesions often heal within 10 to 14 days with no scarring. There is no definite paraclinical test for the diagnosis of RAS [3, 4]. Thus, RAS is often diagnosed according to patient history and clinical manifestations. Taking a precise medical history can rule out the Crohn’s disease, Celiac disease, cyclic neutropenia, HIV infection, and Behcet’s syndrome.
Currently, the treatment of RAS is non-specific and empirical in most cases, and none of the current treatments can lead to permanent recovery. Treatment of recurrent aphthous ulcers is performed aiming to alleviate the symptoms, accelerate the healing process, and decrease the frequency of recurrence [5]. Treatment should be promptly started in the first hours following development of lesions. Identification and elimination of predisposing factors may decrease the frequency of recurrence. Analgesics, local anesthetic agents, anti-inflammatory drugs, anti-septic drugs, steroids, sucralfate, tetracycline, and silver nitrate may be prescribed for local and topical application [6].
Immune system regulators such as dapsone, thalidomide, cyclosporine A, interferon alpha, colchicine, prednisolone, pentoxifylline, methotrexate, tumor necrosis factor antagonists, and azathioprine may also be effective in patients with resistant forms of RAS, or in case of RAS accompanied by a systemic condition. In general, RAS lesions have a favorable prognosis, and often heal spontaneously within a couple of days [6].
Triamcinolone acetonide is a moderate to strong corticosteroid which is derived from prednisolone fluorinate. Its mucoadhesive paste is used as an adjunct for temporary alleviation of symptoms in oral and gingival inflammatory conditions such as RAS [7]. Its mouthwash and crème forms may be used for oral mucosal lesions. Its mouthwash, however, has a short contact time with the lesions, compared with other forms of medication. Crèmes can be easily applied; however, they are also easily washed-out during speech, mastication, and tongue movements. Addition of a mucoadhesive polymer to its crème formulation can prolong its contact time. Thus, triamcinolone mucoadhesive paste was developed for prolonged adhesion. Triadent is a triamcinolone mucoadhesive paste (0.1%) available in the Iranian market [7].
It should be noted that high substantivity of mucoadhesive materials increases the risk of systemic absorption of corticosteroids, which can lead to complications following frequent long-term use [8]. Considering the systemic side effects and complications of steroids such as suppression of the hypothalamus-pituitary-adrenal axis, epithelial atrophy, hyper-pigmentation, candidiasis, and acne, this study aimed to compare the effects of Triadent and Ora-Aid mucoadhesive patch for treatment of minor aphthous ulcers.
Materials and Methods
This clinical trial was conducted at the Oral Medicine Department of School of Dentistry, Yazd University of Medical Sciences between 2020 and 2021. The study protocol was approved by the ethics committee of the university (IR.SSU.REC.1399.314), and registered in the Iranian Registry of Clinical Trials (IRCT20210306050606N1).
Trial design:
A randomized single-blind clinical trial was conducted in which one group received Triadent triamcinolone mucoadhesive paste and the other group received Ora-Aid mucoadhesive patch for treatment of RAS ulcers. The results were reported in accordance with the Consolidated Standards of Reporting Trials.
Participants, eligibility criteria, and settings:
The inclusion criteria were age between 20-40 years, no more than 1 day had to be passed since the development of minor aphthous ulcers, no history of using any other treatment for the aphthous ulcer, no systemic disease related to development of RAS, absence of ulcers on the tonsils and posterior third of the oral cavity, and willingness for participation in the study and signing informed consent forms.
It is worth mentioning that patients were included in this study who had no suspected sign/symptom of any systemic condition related to RAS according to the requested necessary laboratory tests and based on a detailed medical history and clinical examination, and were diagnosed with idiopathic RAS.
The exclusion criteria were consumption of another medication simultaneous with the treatment of RAS, no or irregular use of the prescribed medication as self-reported by patient, not showing up for the recall session, and symptoms of Behcet’s disease (RAS ulcers, recurrent genital ulcers, cutaneous lesions, and ocular lesions).
The sample consisted of 40 eligible patients with RAS presenting to the Oral Medicine Department of Yazd Shahid Sadoughi University of Medical Sciences in 2020-2021.
Interventions:
After comprehensive clinical examination and taking a precise medical history, eligible patients with a confirmed diagnosis of RAS were enrolled. After obtaining written informed consent from the patients, their age, gender, size of lesion, and severity of pain and burning sensation were recorded in a predesigned checklist. The size of lesions was recorded as the largest diameter of the wound. For this purpose, 1 mm grade paper was placed next to the lesions (Figure 1). The level of pain and burning sensation of lesions was quantified by using a visual analog scale (VAS) [9, 10].The patients were instructed on how to use a 100-mm VAS and record their pain level and burning sensation. Score 0 indicated no pain and score 100 indicated maximum pain imaginable. The patients were randomly assigned to two groups by using a table of random numbers. An assistant assigned either medication A or medication B to each patient using a table of random numbers. The Triadent triamcinolone acetonide (0.1%) mucoadhesive paste (Raha Pharmaceuticals, Iran) was used for 20 patients in group A while Ora-Aid mucoadhesive patch (TBM, South Korea) was used for 20 patients in group B. It should be noted that the medication and written instructions on how to use it were placed in identical boxes with a specific code and were delivered to patients by an assistant. The patients in the Triadent group were instructed to first gently dry the mucosal wound surface and then apply some medication over the aphthous ulcer by an applicator. Next, they had to remain their mouth open for 2 minutes. The patients were instructed to apply the medication 3 times/day after meals and refrain from eating and drinking for up to 1 hour after that. Duration of use of medications was determined according to the manufacturer’s instructions. The patients in the Ora-Aid group were instructed to first rinse the ulcer with saline, and then select the proper size mucoadhesive patch, dry the ulcer with a gauze, and apply the patch over the ulcer and compress it for 5-10 seconds to stick to the wound. The patients were recalled after 3, 5, and 7 days following initiation of treatment [11], and their level of pain and burning sensation was assessed by a VAS. Also, the wound size was measured again as explained earlier. Duration of recovery was also recorded. Patients with several aphthous lesions were instructed to use the medication for all ulcers but the largest and most accessible lesion was included in the study.
Sample size calculation:
The sample size was calculated to be 20 in each of the two groups according to a previous study [12] assuming alpha=5%, and study power of 80% using the following formula:
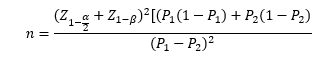
Interim analyses and stopping guidelines:
No interim analyses were performed and no stopping guidelines were established.
Randomization:
The patients were randomly assigned to two groups by using a table of random numbers. An assistant assigned either medication A or medication B to each patient using a table of random numbers. The medications along with their written instructions for use were supplied in similar packages and were randomly delivered to patients.
Blinding:
The assistant and the patients were blinded to their group allocation.
Figure 1. Measuring the largest diameter (length) of a minor aphthous ulcer
Statistical analysis:
The qualitative data were compared between the two groups by the Chi-square test. The quantitative data were compared between the two groups by the t-test. The Mann-Whitney test was applied to compare ordinal variables between the two groups, and repeated measures ANOVA was applied for within-group and between-group comparisons. The Friedman test was also used to analyze the reduction in pain and burning sensation over time. All statistical analyses were carried out using SPSS version 25 (SPSS Inc., IL, USA) at 0.05 level of significance.
Results
The sample consisted of 40 patients including 16 males and 24 females. There were 8 males (40%) and 12 females (60%) in each group. Thus, the two groups had no significant difference in gender distribution as shown by the Chi-square test (P=1.00). The mean age of the patients was 29.40±6.38 years (range 15 to 40 years). The mean age was 30.35±6.07 years in the Triadent group and 28.45±6.70 years in the Ora-Aid group with no significant difference between them as shown by the t-test (P=0.354). Table 1 presents the mean maximum diameter of ulcers (in millimeters) at different time points in the two groups. As shown by the Wilcoxon test, the mean diameter of ulcers was not significantly different between the two groups at 1 and 3 days (P>0.05). However, at 5 (P=0.019) and 7 (P=0.023) days, the mean diameter of the ulcers was significantly smaller in the Ora-Aid group.
Table 1. Mean maximum diameter of ulcers (in millimeters) at different time points in the two groups
Table 2 presents the mean severity of pain and burning sensation (VAS scores). The Mann-Whitney test showed no significant difference in this regard between the two groups at 1, 5, and 7 days (P>0.05). However, the VAS pain and burning score was significantly lower in the Triadent group at 3 days (P=0.015).
Repeated measures ANOVA showed a significant difference between the two groups in the trend of reduction in diameter of ulcers over time (P=0.037, Figure 2).
Table 2. Mean severity of pain and burning sensation (VAS scores) in the two groups
According to the Mann-Whitney test (Table 3), the mean reduction in the diameter of ulcers on day 3 (between days 1 to 3) was significantly greater in the Ora-Aid group than in the Triadent group (P=0.000). However, the mean reduction in diameter of ulcers on day 5 (between 3-5 days) and on day 7 (between 5 to 7 days) was significantly greater in the Triadent group (P=0.000).
The Friedman test showed that the reduction in severity of pain and burning sensation over time was significantly different between the two groups (P=0.022, Figure 3). According to the Mann-Whitney test (Table 4), the mean reduction in pain and burning VAS score was significantly greater in the Ora-Aid group at 3 (between days 1 to 3), 5 (between days 3 to 5), and 7 (between days 5 to 7) days (P=0.000 for all).
Figure 2. Changes in diameter of ulcers over time in the two groups
Table 3. Comparison of the mean reduction in diameter of ulcers at different time points between the two groups
Table 4. Comparison of the mean reduction in pain and burning VAS score at different time points between the two groups
Discussion
Mucoadhesive patches have been introduced as a novel controlled targeted drug delivery system in the field of dentistry [12]. This study compared the effects of Triadent and Ora-Aid mucoadhesive patch on pain severity, burning sensation, and size of minor aphthous ulcers. The present study is among the very first to assess the efficacy of Ora-Aid mucosal adhesive in Iran. Ora-Aid (TBM, Gwangju, Korea) is a mixture of polymers and vitamin E. It is a non-eugenol protective dressing composed of hydrophilic high-density polymers including hydroxy ethyl cellulose according to the latest pharmaceutical standards of South Korea and the United States Pharmacopeia, encapsulated in water-insoluble mucoadhesive synthetic cellulose. It also contains vitamin E which has wound healing and homeostatic effects. The adhesive surface of Ora-Aid is placed directly on the oral mucosa, inducing the oral mucosa to form a protective layer. It aids effective hemostasis, and provides physical protection against foods, bacterial irritants, and cigarette smoke [13]. It is a possible alternative to topical corticosteroids such as triamcinolone acetonide.
Several factors may be used for assessment of treatment efficacy such as reduction in pain and burning sensation, reduction of ulcer size, reduction of number of inflammatory cells in microscopic assessment, and reduction of the number of ulcers [14-16]. In the present study, pain and burning sensation and size of ulcers were evaluated. Pain and burning sensation were evaluated by using a VAS, which is highly reliable for acute and chronic pains and oral symptoms such as ulcers [9, 10].
Diameter of the largest ulcer was measured in the present study, which is a highly accurate and frequently used technique [10, 17]. However, Deshmukh and Bagewadi [18] used a sterile Vernier caliper for measurement of ulcer diameter which is less accurate than the present technique. Since the recovery period of RAS is 7 to 10 days, the treatment course was considered to be 7 days in the present study similar to many previous studies [17, 18]. Fani et al. [7] only evaluated the parameters on day 7, which is less accurate compared with the present methodology [19]. Different medication forms may be used for treatment of ulcers including syrup, creme, mucoadhesive paste, and mouthwash [20]. In the present study, triamcinolone mucoadhesive paste was used similar to studies by Fani et al. [7], Ahadian et al. [17], Mehdipour et al. [21], and Babadi and Poodeh [10], and was compared with Ora-Aid mucoadhesive patch, which is a novel treatment strategy. Unlike Triadnet, which does not have a pleasant taste, Ora-Aid mucoadhesive patch does not have an unpleasant taste and is better accepted by patients. Fani et al. [7], Mehdipour et al. [21], and Babadi and Poodeh [10] used Triadent in comparison with phenytoin mouthwash, zinc-containing mouthwash, and Salvizan gel, which were different from the methodology of the present study. The nature of the two treatments was not the same in the abovementioned studies; thus, an accurate comparison could not be made. However, in the present study, two mucoadhesive substances were compared in different forms, which is a strength, and enables more accurate comparison of their efficacy, similar to a study by Ahadian et al. [17].
In the current study, Ora-Aid was more effective in reduction of pain and burning sensation while Triadent was more effective in reduction of size of ulcers. Babadi and Poodeh [10] reported that Triadent effectively decreased the diameter of ulcers, which was in agreement with the present results. Trident has a paste-like consistency and cannot completely cover the wound; thus, it is partially rinsed off by the saliva. This statement may explain why Ora-Aid was more effective in reduction of pain and burning sensation. This result was similar to the findings of Rodrigues et al. [13] who reported that Ora-Aid mucoadhesive patch was effective for postoperative pain reduction after gingival grafting. Trident is a moderate to strong corticosteroid and because of its strong anti-inflammatory properties, it is expected to be more effective in reduction of size of ulcers.
To the best of the authors’ knowledge, this study is the first to examine the efficacy of Ora-Aid mucoadhesive patches for aphthous ulcers. Therefore, lack of similar studies due to the novelty of the topic is one of the main limitations of the present study. Another limitation of this study was difficult patient recruitment due to the COVID-19 pandemic. In addition, inter-individual differences in healing power and immune system capacity can serve as a confounder and could not be controlled for in the present study, which was another limitation. Future studies with a larger sample size are required to achieve more reliable results.
Conclusion
Both Triadent and Ora-Aid significantly decreased pain and burning sensation and size of minor aphthous lesions. However, Triadent was significantly more effective for reduction of size of lesions while Ora-Aid was significantly more effective for reduction of pain and burning sensation. Thus, Ora-Aid may be used as an alternative to Triadent for treatment of RAS.
Acknowledgement
The authors would like to thank the Vice-Chancellor for Technology and Research of Shahid Sadoughi University of Medical Sciences in Yazd for approving and supporting this research project (Grant no. 1116).
Type of Study: Randomized Clinical Trial |
Subject:
Oral medicine
References
1. Molania T, Malekzadeh Shafaroudi A, Saeedi M, Moosazadeh M, Valipour F, Rostamkalaei SS, et al. Evaluation of cinnamaldehyde mucoadhesive patches on minor recurrent aphthous stomatitis: a randomized, double-blind, placebo-controlled clinical trial. BMC Oral Health. 2022 Jun;22(1):235. [DOI:10.1186/s12903-022-02248-5] [PMID] []
2. Abbasi F, Rasoulzadeh Z, Yavari A. The effect of sage (Salvizan gel) compared to triamcinolone acetonide on the treatment of recurrent aphthous stomatitis: a double-blinded randomized clinical trial. BMC Oral Health. 2023 Mar;23(1):157. [DOI:10.1186/s12903-023-02861-y] [PMID] []
3. Edgar NR, Saleh D, Miller RA. Recurrent Aphthous Stomatitis: A Review. J Clin Aesthet Dermatol. 2017 Mar;10(3):26-36
4. Saikaly SK, Saikaly TS, Saikaly LE. Recurrent aphthous ulceration: a review of potential causes and novel treatments. J Dermatolog Treat. 2018 Sep;29(6):542-52. [DOI:10.1080/09546634.2017.1422079] [PMID]
5. Manfredini M, Guida S, Giovani M, Lippolis N, Spinas E, Farnetani F, et al. Recurrent Aphthous Stomatitis: Treatment and Management. Dermatol Pract Concept. 2021 Sep;11(4):e2021099. [DOI:10.5826/dpc.1104a99] [PMID] []
6. Jurge S, Kuffer R, Scully C, Porter SR. Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral Dis. 2006 Jan;12(1):1-21. [DOI:10.1111/j.1601-0825.2005.01143.x] [PMID]
7. Fani MM, Ebrahimi H, Pourshahidi S, Aflaki E, Shafiee Sarvestani S. Comparing the Effect of Phenytoin Syrup and Triamcinolone Acetonide Ointment on Aphthous Ulcers in Patients with Behcet's Syndrome. Iran Red Crescent Med J. 2012 Feb;14(2):75-8.
8. Messadi DV, Younai F. Aphthous ulcers. Dermatol Ther. 2010 May-Jun;23(3):281-90. [DOI:10.1111/j.1529-8019.2010.01324.x] [PMID]
9. Altenburg A, Abdel-Naser MB, Seeber H, Abdallah M, Zouboulis CC. Practical aspects of management of recurrent aphthous stomatitis. J Eur Acad Dermatol Venereol. 2007 Sep;21(8):1019-26. [DOI:10.1111/j.1468-3083.2007.02393.x] [PMID]
10. Babadi F, Poodeh RM: Comparison of the effect of Salvizan Gel with Teriadent in patients with minor aphthous ulcers. The World Family Medicine/Middle East Journal of Family Medicine. 2017;15(6):115-9. [DOI:10.5742/MEWFM.2017.92989]
11. de Menthon M, Lavalley MP, Maldini C, Guillevin L, Mahr A. HLA-B51/B5 and the risk of Behçet's disease: a systematic review and meta-analysis of case-control genetic association studies. Arthritis Rheum. 2009 Oct;61(10):1287-96. [DOI:10.1002/art.24642] [PMID] []
12. Arafa MG, Ghalwash D, El-Kersh DM, Elmazar MM. Propolis-based niosomes as oromuco-adhesive films: A randomized clinical trial of a therapeutic drug delivery platform for the treatment of oral recurrent aphthous ulcers. Sci Rep. 2018 Dec;8(1):18056. [DOI:10.1038/s41598-018-37157-7] [PMID] []
13. Rodrigues PA, Paramashivaiah R, Prabhuji M, Azevedo RG. Optimized Healing of the Donor Wound Area with Ora-Aid, the Miracle Mix Containing Polymers and Vitamin E: A Case Series. RGUHS journal of dental sciences. 2022 March 14(1):42-6. [DOI:10.26715/rjds.14_1_9]
14. Altenburg A, Zouboulis C: Current concepts in the treatment of recurrent aphthous stomatitis. Skin therapy lett 2008, 13(7):1-4.
15. Cui RZ, Bruce AJ, Rogers RS 3rd. Recurrent aphthous stomatitis. Clin Dermatol. 2016 Jul-Aug;34(4):475-81. [DOI:10.1016/j.clindermatol.2016.02.020] [PMID]
16. Sharma D, Garg R. A Comprehensive Review on Aphthous Stomatitis, its Types, Management and Treatment Available. J Dev Drugs 2018, 7(2):1-8.
17. Ahadian H, Akhavankarbasi M, Sabaghzadegan Y. Comparison of the Clinical Effect of Mucoadhesive Cream of Phenytoin and Triamcinolone Acetonide on the Improvement of Minor Aphthous Ulcer. Journal of Mashhad Dental School. 2020, 44(4):317-27.
18. Deshmukh RA, Bagewadi AS. Comparison of effectiveness of curcumin with triamcinolone acetonide in the gel form in treatment of minor recurrent aphthous stomatitis: A randomized clinical trial. Int J Pharm Investig. 2014 Jul;4(3):138-41. [DOI:10.4103/2230-973X.138346] [PMID] []
19. Nolan A, Baillie C, Badminton J, Rudralingham M, Seymour RA. The efficacy of topical hyaluronic acid in the management of recurrent aphthous ulceration. J Oral Pathol Med. 2006 Sep;35(8):461-5. [DOI:10.1111/j.1600-0714.2006.00433.x] [PMID]
20. Kavitha MK, Kumar MR, Singh SJ: Novel mucoadhesive polymers-a review. J Appl Pharm Sci. 2011;1(8):37-42.
21. Mehdipour M, Taghavi Zenooz A, Sohrabi A, Gholizadeh N, Bahramian A, Jamali Z. A comparison of the effect of triamcinolone ointment and mouthwash with or without zinc on the healing process of aphthous stomatitis lesions. J Dent Res Dent Clin Dent Prospects. 2016 Spring;10(2):87-91. [DOI:10.15171/joddd.2016.014] [PMID] []
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




