Volume 10, Issue 4 (12-2025)
J Res Dent Maxillofac Sci 2025, 10(4): 310-320 |
Back to browse issues page
Ethics code: IR.SBMU.DRC.REC.1399.131
Clinical trials code: 20211123053154N1
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Esmaeilzadeh M, Eghbali A, Ansari G, Fallahinejad Ghajari M, Soleymani A, Yavarifar J et al . Efficacy of Intravenous Propofol-Fentanyl versus Ketamine-Midazolam Combinations for Procedural Sedation of Uncooperative 2-6 Year-Old Pediatric Dental Patients: A Randomized Clinical Trial. J Res Dent Maxillofac Sci 2025; 10 (4) :310-320
URL: http://jrdms.dentaliau.ac.ir/article-1-738-en.html
URL: http://jrdms.dentaliau.ac.ir/article-1-738-en.html
Mohammad Esmaeilzadeh1 
 , Ahmad Eghbali2
, Ahmad Eghbali2 
 , Ghassem Ansari1
, Ghassem Ansari1 
 , Masoud Fallahinejad Ghajari1
, Masoud Fallahinejad Ghajari1 
 , Ali Soleymani1
, Ali Soleymani1 
 , Jina Yavarifar3
, Jina Yavarifar3 
 , Leila Eftekhar *4
, Leila Eftekhar *4 


 , Ahmad Eghbali2
, Ahmad Eghbali2 
 , Ghassem Ansari1
, Ghassem Ansari1 
 , Masoud Fallahinejad Ghajari1
, Masoud Fallahinejad Ghajari1 
 , Ali Soleymani1
, Ali Soleymani1 
 , Jina Yavarifar3
, Jina Yavarifar3 
 , Leila Eftekhar *4
, Leila Eftekhar *4 

1- Department of Pediatric Dentistry, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Anesthesiology Research Center, Mofid Children's Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3- Private Dentistry Practice, Tehran, Iran
4- Department of Pediatric Dentistry, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,leila.eftekhar.a@gmail.com
2- Anesthesiology Research Center, Mofid Children's Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
3- Private Dentistry Practice, Tehran, Iran
4- Department of Pediatric Dentistry, School of Dentistry, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,
Full-Text [PDF 393 kb]
(8 Downloads)
| Abstract (HTML) (13 Views)
Full-Text: (2 Views)
Abstract
Background and Aim: This study aimed to evaluate the efficacy and safety of intravenous propofol-fentanyl versus ketamine-midazolam combinations for sedation of 2-6-year-old uncooperative dental patients.
Materials and Methods: This triple-blind crossover randomized clinical trial included 24 (13 boys and 11 girls) healthy (ASA I) uncooperative (Frankl I) 2-6-year-old (mean age: 3.7±1.1 years) children who were randomly divided into two groups. The first group (AB) received intravenous propofol (2 mg/kg) in addition to fentanyl (1 µg/kg) in the first session and ketamine (2 mg/kg) along with midazolam (0.2 mg/kg) in the second session. The second group (BA) received the two drug combinations in a reverse order to allow testing the potential sequence effect. Oral midazolam (0.3 mg/kg) was administered as premedication. Changes in hemodynamic indices and reactions to dental stimulations were recorded using the monitoring device and Houpt scale, respectively. Data were analyzed using the Pearson Chi-Square test, independent t-test, and Mann-Whitney U test (alpha=0.05).
Results: There were no significant differences in hemodynamic indices, namely the SpO2 (P=0.179) and heart rate (HR) (P=0.062), and overall behavior (P=0.378) of the two groups during the treatment phases, including catheter insertion, local anesthetic injection, and at 15 and 30 minutes following the onset of dental treatment. However, the recovery time was significantly longer in the ketamine-midazolam than the propofol-fentanyl group (P=0.00).
Conclusion: Based on the results of this study, the use of propofol-fentanyl combination for behavioral control of uncooperative children provided better sedation quality and scores, and lower hemodynamic changes compared to ketamine-midazolam, although this difference was not statistically significant.
Keywords: Fentanyl; Administration, Intravenous; Ketamine; Pediatric Dentistry; Propofol
Introduction
Materials and Methods: This triple-blind crossover randomized clinical trial included 24 (13 boys and 11 girls) healthy (ASA I) uncooperative (Frankl I) 2-6-year-old (mean age: 3.7±1.1 years) children who were randomly divided into two groups. The first group (AB) received intravenous propofol (2 mg/kg) in addition to fentanyl (1 µg/kg) in the first session and ketamine (2 mg/kg) along with midazolam (0.2 mg/kg) in the second session. The second group (BA) received the two drug combinations in a reverse order to allow testing the potential sequence effect. Oral midazolam (0.3 mg/kg) was administered as premedication. Changes in hemodynamic indices and reactions to dental stimulations were recorded using the monitoring device and Houpt scale, respectively. Data were analyzed using the Pearson Chi-Square test, independent t-test, and Mann-Whitney U test (alpha=0.05).
Results: There were no significant differences in hemodynamic indices, namely the SpO2 (P=0.179) and heart rate (HR) (P=0.062), and overall behavior (P=0.378) of the two groups during the treatment phases, including catheter insertion, local anesthetic injection, and at 15 and 30 minutes following the onset of dental treatment. However, the recovery time was significantly longer in the ketamine-midazolam than the propofol-fentanyl group (P=0.00).
Conclusion: Based on the results of this study, the use of propofol-fentanyl combination for behavioral control of uncooperative children provided better sedation quality and scores, and lower hemodynamic changes compared to ketamine-midazolam, although this difference was not statistically significant.
Keywords: Fentanyl; Administration, Intravenous; Ketamine; Pediatric Dentistry; Propofol
Introduction
Fear of dentistry and consequent behavioral management problems are among the most common factors that prevent children from receiving high-quality dental services. This fear is closely related to the severity of dental caries and the volume of therapeutic interventions required [1]. Children's anxiety and fear of dentistry usually peak during anesthetic injections, therapeutic intervention, and separation from their parents [2]. The fear and anxiety stimulate the child's sympathetic and parasympathetic systems and ultimately increase the heart rate (HR), blood pressure, and respiratory rate, and cause behavioral and psychological disorders [3]. Studies show that dentists and parents of children prefer non-pharmacological behavioral management techniques such as behavioral shaping, positive reinforcement, and even voice control. However, in many cases, treatment of uncooperative anxious children and those with disabilities is only possible through pharmacological measures including sedation and/or general anesthesia [4]. In dental sedation, partial depression of the central nervous system is achieved to ensure provision of efficient and effective treatment in a calm manner while maintaining the vital reflexes. Therefore, moderate to deep sedation along with local anesthesia is advocated as a good alternative to general anesthesia for dental treatment of ASA Class I and II uncooperative children [4]. An ideal technique and drug for sedation should have a rapid onset of action, optimal stability during therapeutic intervention, and rapid recovery [4]. It has been shown that intravenous sedation with a combination of drugs could act as an effective and safe approach for behavioral control of uncooperative children [5]. Various drug combinations have been used for intravenous sedation, with optimal efficacy while minimizing side effects through reduced individual doses [6]. Meanwhile, it appears that midazolam, ketamine, propofol, and fentanyl are among the most commonly applied medications in dental sedation of uncooperative children for dental treatments [7]. Midazolam, a water-soluble sedative-hypnotic benzodiazepine, is presented as a safer medication with fewer side effects compared to most hypnotics (such as chloral hydrate and barbiturates). However, potentially severe psychological agitation in some patients has limited the use of monotherapy with midazolam [8]. Ketamine produces dissociative anesthesia and has an analgesic action [9]. It is routinely used to initiate general anesthesia and is used for sedation in lower doses. A long recovery period along with unpleasant dreams and some degrees of hallucination, are among the disadvantages of this medication [10]. Propofol, on the other hand, is a relatively new intravenous hypnotic drug with a relatively unknown mechanism of action. Propofol is the gold standard for the induction and maintenance of anesthesia and procedural sedation. One of the advantages of propofol is rapid recovery even after long periods of anesthesia and conscious sedation [11]. Fentanyl is a fast-acting, short-lasting narcotic (opioid) with stronger analgesic effects compared to morphine. It has fewer gastrointestinal side effects (nausea and vomiting), while at high doses, fentanyl has the risk of bradycardia, chest muscle stiffness, and respiratory depression [10]. Opioids have recently been used for sedation and general anesthesia, which results in a better painless state and provide a more stable cardiovascular state. Therefore, opioids can be used in combination with propofol to provide better analgesia in patients undergoing painful procedures [12]. Fentanyl is used in combination with propofol as an adjuvant to allow for the effective use of propofol at lower doses [13]. Nowadays, sedation techniques have been significantly improved by the use of new drug combinations, advances in monitoring technologies, increased clinical skills of operators, and community acceptance. The earlier sedative agents, including chloral hydrate, diazepam, morphine, pethidine, and pentobarbital have been abandoned due to their significant clinical side effects and long recovery time. The new generation of sedative drugs includes fast-acting drugs such as fentanyl, benzodiazepines, ketamine, and nitrous oxide. However, propofol and etomidate have gained increasing popularity for routine use as both offer a rapid onset, shorter recovery, and minimal clinical side effects [14]. Despite many clinical studies in the field of sedation, the appropriate and ideal drug combination has not yet been agreed upon as an "ideal or golden combination" for proper and safe sedation in pediatric dentistry. More recent investigations focused on the clinical effects of various drug combinations to achieve maximum sedative effects along with minimal clinical side effects [15]. Thus, this comparative study was designed to evaluate the sedation effects and clinical complications of intravenous administration of propofol-fentanyl and ketamine-midazolam combinations in dental treatment of uncooperative children aged 2 to 6 years.
Materials and Methods
Materials and Methods
This study was performed at the Pediatric Sedation Unit of Shahid Beheshti Dental School between 2022 and 2023. The study protocol was approved by the ethics committee of Shahid Beheshti University of Medical Sciences (Ethics code: IR.SBMU.DRC.REC.1399.131) and registered in the Iranian Registry of Clinical Trials (IRCT code: 20211123053154N1).
Trial design
A triple-blind randomized crossover clinical trial was designed in which the two groups of children received intravenous propofol-fentanyl and ketamine-midazolam combinations for sedation in reverse orders. The results were reported according to the guidelines of the Consolidated Standards of Reporting Trials.
Participants, eligibility criteria, and settings:
The inclusion criteria were: A) uncooperative 2-6-year-old children with completely negative behavior according to Frankl’s behavior rating scale. [17], B) ASA Class I health status, and C) patients requiring at least 2 sessions of similar dental treatments under local anesthesia.
The exclusion criteria were (A) common cold or any respiratory obstruction, and (B) any medical condition, such as allergy to sedative agents or a condition that prohibits the use of such drugs.
The sample consisted of 24 uncooperative children aged 2-6 years referred to the Pediatric Sedation Unit of Shahid Beheshti Dental School, who were selected by convenience sampling.
Interventions
Parents of all participating children signed informed consent forms prior to enrollment of their children. Before the dental treatment, the children underwent dental and medical examinations.
Necessary pre-treatment instructions were given in written and verbal forms upon the child's admission, including observation of NPO for 6 hours for solid foods and milk, and 3 hours for drinking clear liquids. The selected children were randomly divided into two treatment groups. Accordingly, 12 children were assigned to the AB treatment group and 12 to the BA treatment group. In the AB group, intravenous propofol-fentanyl (drug regimen A) was administered in the first treatment session, and intravenous ketamine-midazolam (drug regimen B) was given in the second session. The BA group received the two combinations in a reverse order. The two sessions of dental treatment were designed in such a way that they were similar and comparable in terms of type of session and duration of treatment.
All participating children received 0.3 mg/kg oral midazolam as a pre-sedation medication in every session. After a 30-minute waiting time, the child received the intravenous drug through the catheter. Children on the drug regimen A were first injected with a combination of fentanyl (1µ g
The recorded vital signs included the HR and blood oxygen saturation (SpO2) during the treatment process and at the recording steps.
At the end of treatment, the children were discharged following the observation of discharge signs and symptoms including stable cardiovascular and respiratory systems and active protective responses.
Outcomes (primary and secondary)
HR, SpO2, and Houpt scale were the primary outcomes in this study. There was no secondary outcome.
Sample size calculation
The following formula was used to calculate the sample size:
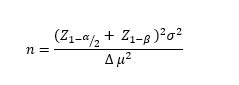
Accordingly, assuming the type I error (α)=0.05 ( Z 1- α 2 Z 1- β
Interim analyses and stopping guideline
None.
Randomization
Random allocation of patients was performed by tossing a coin. For allocation concealment, sealed envelopes with a random sequence were used, which were randomly delivered to each patient in the first treatment session by the assistant.
Blinding
As the administered drug combinations were prepared by the anesthesiologist in charge, neither the operator (dentist), nor the children or their parents were aware of the composition or their order of administration. The person recording the data was also blinded to the group allocations. Thus, the study had a triple-blind design.
Statistical analysis
Evaluation and comparison of the collected data were performed using the Pearson Chi-Square and independent t-tests to analyze differences in sex, age, and weight. One-sample Kolmogorov-Smirnov test was used to analyze the normality of SpO2 and HR, and an independent t-test was used to analyze differences in this regard between the two groups. Also, the Man-Whitney U test was used to analyze the Houpt scale results. One-sample Kolmogorov-Smirnov test was used to analyze the normality of recovery time data, and independent t-test was applied to compare the groups in this regard.
Results
Trial design
A triple-blind randomized crossover clinical trial was designed in which the two groups of children received intravenous propofol-fentanyl and ketamine-midazolam combinations for sedation in reverse orders. The results were reported according to the guidelines of the Consolidated Standards of Reporting Trials.
Participants, eligibility criteria, and settings:
The inclusion criteria were: A) uncooperative 2-6-year-old children with completely negative behavior according to Frankl’s behavior rating scale. [17], B) ASA Class I health status, and C) patients requiring at least 2 sessions of similar dental treatments under local anesthesia.
The exclusion criteria were (A) common cold or any respiratory obstruction, and (B) any medical condition, such as allergy to sedative agents or a condition that prohibits the use of such drugs.
The sample consisted of 24 uncooperative children aged 2-6 years referred to the Pediatric Sedation Unit of Shahid Beheshti Dental School, who were selected by convenience sampling.
Interventions
Parents of all participating children signed informed consent forms prior to enrollment of their children. Before the dental treatment, the children underwent dental and medical examinations.
Necessary pre-treatment instructions were given in written and verbal forms upon the child's admission, including observation of NPO for 6 hours for solid foods and milk, and 3 hours for drinking clear liquids. The selected children were randomly divided into two treatment groups. Accordingly, 12 children were assigned to the AB treatment group and 12 to the BA treatment group. In the AB group, intravenous propofol-fentanyl (drug regimen A) was administered in the first treatment session, and intravenous ketamine-midazolam (drug regimen B) was given in the second session. The BA group received the two combinations in a reverse order. The two sessions of dental treatment were designed in such a way that they were similar and comparable in terms of type of session and duration of treatment.
All participating children received 0.3 mg/kg oral midazolam as a pre-sedation medication in every session. After a 30-minute waiting time, the child received the intravenous drug through the catheter. Children on the drug regimen A were first injected with a combination of fentanyl (1
The recorded vital signs included the HR and blood oxygen saturation (SpO2) during the treatment process and at the recording steps.
At the end of treatment, the children were discharged following the observation of discharge signs and symptoms including stable cardiovascular and respiratory systems and active protective responses.
Outcomes (primary and secondary)
HR, SpO2, and Houpt scale were the primary outcomes in this study. There was no secondary outcome.
Sample size calculation
The following formula was used to calculate the sample size:
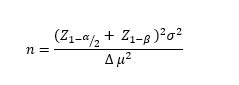
Accordingly, assuming the type I error (α)=0.05 (
Interim analyses and stopping guideline
None.
Randomization
Random allocation of patients was performed by tossing a coin. For allocation concealment, sealed envelopes with a random sequence were used, which were randomly delivered to each patient in the first treatment session by the assistant.
Blinding
As the administered drug combinations were prepared by the anesthesiologist in charge, neither the operator (dentist), nor the children or their parents were aware of the composition or their order of administration. The person recording the data was also blinded to the group allocations. Thus, the study had a triple-blind design.
Statistical analysis
Evaluation and comparison of the collected data were performed using the Pearson Chi-Square and independent t-tests to analyze differences in sex, age, and weight. One-sample Kolmogorov-Smirnov test was used to analyze the normality of SpO2 and HR, and an independent t-test was used to analyze differences in this regard between the two groups. Also, the Man-Whitney U test was used to analyze the Houpt scale results. One-sample Kolmogorov-Smirnov test was used to analyze the normality of recovery time data, and independent t-test was applied to compare the groups in this regard.
Results
Participant flow
This study was performed on 24 children, including 11 girls and 13 boys, with a mean age of 3.7±1.1 years and a mean weight of 16.1±3.8 kg. Based on the results of the Pearson Chi-Square test and independent t-test, the difference between the two treatment groups of AB and BA was not significant in terms of gender (P=0.682), age (P=0.537), and weight (P=0.283). Figure 1 shows the CONSORT flow diagram of patient selection and allocation.
Harms
No patients were harmed during the study.
SpO2
As shown in Table 1, the highest mean SpO2 was recorded in the AB group at the time of injection (T2) (99%±1.1%), and the lowest was recorded at the time of discharge (T5) (97.75%±1.1%) in the first phase of sedation treatment (F). In the BA group, the highest mean SpO2 was recorded at 15 minutes following the treatment onset (T3) (99.75% ± 0.45%) and the lowest at baseline (T0) (98%±1.27%).
This study was performed on 24 children, including 11 girls and 13 boys, with a mean age of 3.7±1.1 years and a mean weight of 16.1±3.8 kg. Based on the results of the Pearson Chi-Square test and independent t-test, the difference between the two treatment groups of AB and BA was not significant in terms of gender (P=0.682), age (P=0.537), and weight (P=0.283). Figure 1 shows the CONSORT flow diagram of patient selection and allocation.
Harms
No patients were harmed during the study.
SpO2
As shown in Table 1, the highest mean SpO2 was recorded in the AB group at the time of injection (T2) (99%±1.1%), and the lowest was recorded at the time of discharge (T5) (97.75%±1.1%) in the first phase of sedation treatment (F). In the BA group, the highest mean SpO2 was recorded at 15 minutes following the treatment onset (T3) (99.75% ± 0.45%) and the lowest at baseline (T0) (98%±1.27%).
Figure 1. CONSORT flow diagram of patient selection and allocation
The highest mean SpO2 in the AB group was at the time of injection (T2) (99.58% ± 0.67%), and the lowest was recorded at baseline (T0) (98.25% ± 0.6%) in the second phase of sedation treatment (S). In the BA group, the highest mean SpO2 was recorded at the time of injection (T2) (99.58% ± 0.67%) and the lowest at baseline (T0) (97.9% ± 0.99%).
Heart Rate
As reported in Table 2, the highest mean HR in the AB group in the (F) treatment phase was at the time of discharge (T5) (137.4±24 beats/minute), and the lowest value was recorded at baseline (T0) (106.4±13 beats/minute). In the BA group, the highest mean HR was at 15 minutes following the treatment onset (T3) (133.1± 15 beats/minute) and the lowest at baseline (T0) (110.6±14 beats/minute).
In the AB group, the highest mean HR was recorded at 15 minutes (T3) (141.4±12 beats/minute) and the lowest at baseline (T0) (111±12 beats/minute) in the second phase of treatment (S). In the BA group, the highest mean HR was recorded at the time of discharge (T5) (135.7±11 beats/minute) and the lowest at baseline (T0) (111±11 beats/minute).
The one-sample Kolmogorov-Smirnov test confirmed the normality of data distribution for SpO2 and HR following the evaluation of carryover effect. The carryover effect for the two drug regimens of A and B was not statistically significant (P˃0.05). In other words, there was no obvious carryover effect between the clinical data of the two drug regimens (A and B), and this finding also indicated the adequacy of the washout period. The treatment effect was not significant either (P˃0.05). Therefore, distribution of data in the two treatment groups was normal and thus independent t-test was used to examine these effects between the two treatment groups (AB and BA).
The effect of treatment on SpO2 (P=0.179) and HR (P=0.062) was not significant, indicating no significant difference between the two groups of AB and BA in this regard.
Houpt Scale score
The Houpt Scale of sedation was recorded in details. The overall behavior of children (O) at T2, T3, and T4 during the first (F) and second (S) treatment phases in both the AB and BA groups was dominantly excellent, with no movement and no crying during treatment. At T1, the 4th rank (well; the treatment was difficult but was done) was the most common rank in group AB in the first and second phases while, the most common rank was the 5th rank (very good, treated with a little crying and movement) in the first phase and 3rd rank (moderate, intermittent treatment with hardship was performed) and 4th rank in the second phase in the BA group.
All four components of sleepiness (S), movement (M), crying (C), and overall behavior (O) of the child were ranked to evaluate the effect of treatment using the non-parametric Mann-Whitney test. The treatment effect difference was not statistically significant between the groups in any of the four components (P=0.551 for S, P=0.755 for M, P=0.242 for C, and P=0.378 for O). These findings showed similar effects of both drug regimens on the Houpt behavior rating scale (Table 3).
The mean recovery index (R) in the AB treatment group was 26.67±3.25 minutes in the first phase, and 43.75 ±6.07 minutes in the second phase. These findings were reported for the BA treatment group to be 44.58±4.5 minutes in the first phase of treatment, and 27.7±4.1 minutes in the second phase of treatment. Considering the recovery time, the difference between the two study groups of AB and BA was statistically significant (P=0.000). Therefore, a significant therapeutic difference was observed between the two groups in terms of recovery time index (Table 4).
Heart Rate
As reported in Table 2, the highest mean HR in the AB group in the (F) treatment phase was at the time of discharge (T5) (137.4±24 beats/minute), and the lowest value was recorded at baseline (T0) (106.4±13 beats/minute). In the BA group, the highest mean HR was at 15 minutes following the treatment onset (T3) (133.1± 15 beats/minute) and the lowest at baseline (T0) (110.6±14 beats/minute).
In the AB group, the highest mean HR was recorded at 15 minutes (T3) (141.4±12 beats/minute) and the lowest at baseline (T0) (111±12 beats/minute) in the second phase of treatment (S). In the BA group, the highest mean HR was recorded at the time of discharge (T5) (135.7±11 beats/minute) and the lowest at baseline (T0) (111±11 beats/minute).
The one-sample Kolmogorov-Smirnov test confirmed the normality of data distribution for SpO2 and HR following the evaluation of carryover effect. The carryover effect for the two drug regimens of A and B was not statistically significant (P˃0.05). In other words, there was no obvious carryover effect between the clinical data of the two drug regimens (A and B), and this finding also indicated the adequacy of the washout period. The treatment effect was not significant either (P˃0.05). Therefore, distribution of data in the two treatment groups was normal and thus independent t-test was used to examine these effects between the two treatment groups (AB and BA).
The effect of treatment on SpO2 (P=0.179) and HR (P=0.062) was not significant, indicating no significant difference between the two groups of AB and BA in this regard.
Houpt Scale score
The Houpt Scale of sedation was recorded in details. The overall behavior of children (O) at T2, T3, and T4 during the first (F) and second (S) treatment phases in both the AB and BA groups was dominantly excellent, with no movement and no crying during treatment. At T1, the 4th rank (well; the treatment was difficult but was done) was the most common rank in group AB in the first and second phases while, the most common rank was the 5th rank (very good, treated with a little crying and movement) in the first phase and 3rd rank (moderate, intermittent treatment with hardship was performed) and 4th rank in the second phase in the BA group.
All four components of sleepiness (S), movement (M), crying (C), and overall behavior (O) of the child were ranked to evaluate the effect of treatment using the non-parametric Mann-Whitney test. The treatment effect difference was not statistically significant between the groups in any of the four components (P=0.551 for S, P=0.755 for M, P=0.242 for C, and P=0.378 for O). These findings showed similar effects of both drug regimens on the Houpt behavior rating scale (Table 3).
The mean recovery index (R) in the AB treatment group was 26.67±3.25 minutes in the first phase, and 43.75 ±6.07 minutes in the second phase. These findings were reported for the BA treatment group to be 44.58±4.5 minutes in the first phase of treatment, and 27.7±4.1 minutes in the second phase of treatment. Considering the recovery time, the difference between the two study groups of AB and BA was statistically significant (P=0.000). Therefore, a significant therapeutic difference was observed between the two groups in terms of recovery time index (Table 4).
Table 1. Mean and standard deviation of SpO2 (%) during the first and second phases of treatment in the two groups
Table 2. Mean and standard deviation of HR (beats/minute) during the first and second phases of treatment in the two groups
Table 3. Houpt Behavior scale in the two groups
Table 4. Mean and standard deviation of recovery time (minutes) during the first and second phase of treatment in the two groups
Discussion
Table 2. Mean and standard deviation of HR (beats/minute) during the first and second phases of treatment in the two groups
Table 3. Houpt Behavior scale in the two groups
Table 4. Mean and standard deviation of recovery time (minutes) during the first and second phase of treatment in the two groups
Discussion
Despite extensive studies in the field of drugs suitable for use in sedation, no ideal drug or combination of drugs has yet been introduced as the "golden combination" for sedation via various routes, including the venous route, and no drug combination has yet been agreed upon by clinicians [15]. This study aimed to compare the changes in SpO2, HR, Houpt scale, and recovery time of intravenous propofol-fentanyl versus ketamine-midazolam combinations for procedural sedation of uncooperative 2-6-year-old pediatric dental patients. Midazolam has been introduced as the most common and widely used drug in the group of benzodiazepines for sedation [19]. Midazolam can be combined with other drug groups to increase the therapeutic effects, including increasing the appropriate sedation level, inducing analgesia, and reducing side effects [7]. Monotherapy with ketamine as the drug of choice, in addition to maintaining normal respiratory and cardiovascular function, can cause anesthesia, relative sedation, adequate analgesia, and even amnesia in patients through its dissociative function. It is recommended to either use the minimum dose of this drug or use it in combination with other drugs such as midazolam and atropine [20-22].
Propofol is a new third-generation intravenous hypnotic drug with a relatively unknown mechanism of action. Due to the extreme lipophilic properties of this drug, its onset of action and recovery are very fast [14, 23]. The results of clinical studies have shown that propofol, unlike ketamine, does not have analgesic effects at the usual doses. Therefore, to induce analgesic effects in patients, it is used in combination with other drugs, including new-generation narcotic drugs such as fentanyl and remifentanil [14, 24]. Fentanyl is a new, fast-acting, and short-lasting lipophilic narcotic drug with strong analgesic effects [25]. It has fewer gastrointestinal side effects (nausea and vomiting) than other narcotic drugs. Fentanyl is a weak sedative despite its excellent analgesic effects. Therefore, it should be used with other second or third-generation sedatives for sedation [25]. Thus, comparing the effectiveness and side effects of ketamine/midazolam drug combination as a second-generation sedative/analgesic and acceptable drug regimen in pediatric dental sedation, with propofol as a new third-generation sedative along with fentanyl as a strong analgesic, was the main purpose of designing and conducting this randomized clinical trial on uncooperative children requiring dental treatment under sedation. The results of this study regarding lack of significant negative effects of these two drug combinations on hemodynamic parameters of oxygen saturation and HR, and therefore their safety, are aligned with the findings of Bahrami Gorji et al. [26], Dal et al. [27], Guan et al. [28], Thakur et al. [29], and Khatavkar and Bakhshi [30]. However, according to das Neves et al. [31], the use of propofol/fentanyl significantly reduced HR and resulted in bradycardia; this difference can be attributed to the administration of atropine in the present clinical trial, unlike the study by das Neves et al [31]. Regarding the overall success rate of sedation according to the Houpt scale and evaluation of the overall behavior of children, the success rate of both drug groups was similar and equal to 100%. This conclusion is consistent with the findings of studies by Barkan et al. [32], (94% success for the midazolam/ketamine group), Ghai et al. [33] (98% success for the midazolam/ketamine group), and Ahmed et al. [34] (100% success for the propofol/fentanyl group). In contrast, studies by Soleimanpour et al. [35], (62.5% success for the midazolam/ketamine group), and Majidinejad et al. [36] (45.5% success for the midazolam/ketamine group) reported a much lower success rates, which may be due to differences in success metrics and criteria, dosage and route of administration of drugs, and differences in the design and methodology of the studies. The present results regarding the recovery time index in the two drug groups of propofol/fentanyl and midazolam/ketamine showed a statistically significant difference between the two groups. It means that the midazolam/ketamine drug group had a much longer recovery time than the propofol/fentanyl drug group, which was aligned with the results of Dal et al. [27] and Kramer et al. [37]. The most important reason for this significant difference is the use of ketamine, with long-term recovery being one of its major disadvantages.
Limitations of this study included non-attendance of patients in the second session of dental treatment and limitations regarding access to fentanyl. Also, it was difficult to observe fasting or NPO time of children, which, despite the previous emphasis and training of the parents, was not implemented correctly in some cases causing cancellation of the treatment session. Evaluation of other drug combinations with propofol to find the golden combination and achieve short recovery, stable treatment, and adequate pain control for dental treatments under intravenous sedation is recommended.
Conclusion
Propofol is a new third-generation intravenous hypnotic drug with a relatively unknown mechanism of action. Due to the extreme lipophilic properties of this drug, its onset of action and recovery are very fast [14, 23]. The results of clinical studies have shown that propofol, unlike ketamine, does not have analgesic effects at the usual doses. Therefore, to induce analgesic effects in patients, it is used in combination with other drugs, including new-generation narcotic drugs such as fentanyl and remifentanil [14, 24]. Fentanyl is a new, fast-acting, and short-lasting lipophilic narcotic drug with strong analgesic effects [25]. It has fewer gastrointestinal side effects (nausea and vomiting) than other narcotic drugs. Fentanyl is a weak sedative despite its excellent analgesic effects. Therefore, it should be used with other second or third-generation sedatives for sedation [25]. Thus, comparing the effectiveness and side effects of ketamine/midazolam drug combination as a second-generation sedative/analgesic and acceptable drug regimen in pediatric dental sedation, with propofol as a new third-generation sedative along with fentanyl as a strong analgesic, was the main purpose of designing and conducting this randomized clinical trial on uncooperative children requiring dental treatment under sedation. The results of this study regarding lack of significant negative effects of these two drug combinations on hemodynamic parameters of oxygen saturation and HR, and therefore their safety, are aligned with the findings of Bahrami Gorji et al. [26], Dal et al. [27], Guan et al. [28], Thakur et al. [29], and Khatavkar and Bakhshi [30]. However, according to das Neves et al. [31], the use of propofol/fentanyl significantly reduced HR and resulted in bradycardia; this difference can be attributed to the administration of atropine in the present clinical trial, unlike the study by das Neves et al [31]. Regarding the overall success rate of sedation according to the Houpt scale and evaluation of the overall behavior of children, the success rate of both drug groups was similar and equal to 100%. This conclusion is consistent with the findings of studies by Barkan et al. [32], (94% success for the midazolam/ketamine group), Ghai et al. [33] (98% success for the midazolam/ketamine group), and Ahmed et al. [34] (100% success for the propofol/fentanyl group). In contrast, studies by Soleimanpour et al. [35], (62.5% success for the midazolam/ketamine group), and Majidinejad et al. [36] (45.5% success for the midazolam/ketamine group) reported a much lower success rates, which may be due to differences in success metrics and criteria, dosage and route of administration of drugs, and differences in the design and methodology of the studies. The present results regarding the recovery time index in the two drug groups of propofol/fentanyl and midazolam/ketamine showed a statistically significant difference between the two groups. It means that the midazolam/ketamine drug group had a much longer recovery time than the propofol/fentanyl drug group, which was aligned with the results of Dal et al. [27] and Kramer et al. [37]. The most important reason for this significant difference is the use of ketamine, with long-term recovery being one of its major disadvantages.
Limitations of this study included non-attendance of patients in the second session of dental treatment and limitations regarding access to fentanyl. Also, it was difficult to observe fasting or NPO time of children, which, despite the previous emphasis and training of the parents, was not implemented correctly in some cases causing cancellation of the treatment session. Evaluation of other drug combinations with propofol to find the golden combination and achieve short recovery, stable treatment, and adequate pain control for dental treatments under intravenous sedation is recommended.
Conclusion
Based on the results obtained from the present study, the effect of intravenous administration of propofol/fentanyl was similar to that of midazolam/ketamine on hemodynamic parameters of SpO2 and HR, maintaining them within the normal range. Also, the intravenous sedation effect of propofol/fentanyl was almost the same as that of midazolam/ketamine on behavioral control of uncooperative 2-6-year-old children requiring dental treatment under sedation.
Type of Study: Original article |
Subject:
pediatric
References
1. Slabšinskienė E, Kavaliauskienė A, Žemaitienė M, Vasiliauskienė I, Zaborskis A. Dental Fear and Associated Factors among Children and Adolescents: A School-Based Study in Lithuania. Int J Environ Res Public Health. 2021;18(16). [DOI:10.3390/ijerph18168883] [PMID] []
2. Dean JA, Jones JE, Walker Vinson LA, McDonald RE. McDonald and Avery's Dentistry for the Child and Adolescent. 11th ed. St. Louis, MO: Elsevier; 2022:285-311
3. Venn R, Sigrist C, Rudzki S, D'Cunha NM, Buchhorn R, Koenig J, et al. Mental health consequences of early life stress in children - The autonomic nervous system as a potential target for early detection and intervention? Psychoneuroendocrinology. 2025; 179:107505. [DOI:10.1016/j.psyneuen.2025.107505] [PMID]
4. Ferrazzano GF, Cantile T, Quaraniello M, Iannuzzi M, Palumbo D, Servillo G, Caruso S, Fiasca F, Ingenito A. Effectiveness and Safety of Intravenous Sedation with Propofol in Non-Operating Room Anesthesia (NORA) for Dental Treatment in Uncooperative Paediatric Patients. Children (Basel). 2021 Jul 28;8(8):648. [DOI:10.3390/children8080648] [PMID] []
5. Eghbali Zarch A, Amiri Tehranizadeh N, Ansari G, Fallahinejad Ghajari M. Sedative Effect of Intravenous Propofol-Ketamine and Midazolam Ketamine Combinations for Dental Treatment of Uncooperative 2-6-Year-Old Children: A Clinical Trial. J Res Dent Maxillofac Sci 2025; 10 (2) :125-133. [DOI:10.61186/jrdms.10.2.125]
6. Esmaillian M, Kouhestani S, Azizkhani R, Heydari F, Safavi MR. Dexmedetomidine versus propofol: An effective combination with ketamine for adult procedural sedation: A randomized clinical trial. Am J Emerg Med. 2023 Nov;73:95-101. [DOI:10.1016/j.ajem.2023.08.025] [PMID]
7. Kapur A, Kapur V. Conscious Sedation in Dentistry. Ann Maxillofac Surg. 2018 Jul-Dec;8(2):320-3. [DOI:10.4103/ams.ams_191_18] [PMID] []
8. Miller J. Managing acute agitation and aggression in the world of drug shortages. Ment Health Clin. 2021;11(6):334-46. [DOI:10.9740/mhc.2021.11.334] [PMID] []
9. Amin NM, Sawan Z, Bahri SS, Abdelfattah WM. Inhalation of Sevoflurane Versus Intravenous Ketamine, Midazolam and Propofol For Sedation in Pediatrics Undergoing Upper Gastrointestinal Endoscopy. Zagazig University Medical Journal. 2023 Jul 1;29(4):1135-45.
10. Malamed SJ. Sedation: A guide to patient management. 2017; 6th ed. Mosby, st Louis: 332-333, 344-345,358-364.
11. Paramsothy J, Gutlapalli SD, Ganipineni VDP, Mulango I, Okorie IJ, Arrey Agbor DB, Delp C, Apple H, Kheyson B, Nfonoyim J, Isber N, Yalamanchili M. Propofol in ICU Settings: Understanding and Managing Anti-Arrhythmic, Pro-Arrhythmic Effects, and Propofol Infusion Syndrome. Cureus. 2023 Jun 15;15(6):e40456. [DOI:10.7759/cureus.40456]
12. Azarfar A, Ravanshad Y, Golsorkhi M, Zahiri E, Gharavi Fard M, Akhondi M, Ghodsi A, Ravanshad S. Comparison of Propofol-Fentanyl with Midazolam-Ketamine Combination in Pediatric Patients Undergoing Kidney Biopsy. Iran J Kidney Dis. 2022 Jul;16(4):246-51.
13. Saylan S, Akbulut UE. A comparison of ketamine-midazolam combination and propofol-fentanyl combination on procedure comfort and recovery process in pediatric colonoscopy procedures. Pak J Med Sci. 2021 Mar-Apr;37(2):483-8. [DOI:10.12669/pjms.37.2.2787] [PMID] []
14. Cappellini I, Bavestrello Piccini G, Campagnola L, Bochicchio C, Carente R, Lai F, et al. Procedural Sedation in Emergency Department: A Narrative Review. Emerg. Care Med. 2024, 1, 103-136. [DOI:10.3390/ecm1020014]
15. Jaikaria A, Thakur S, Singhal P, Chauhan D, Jayam C, Syal K. A Comparison of Oral Midazolam-ketamine, Dexmedetomidine-fentanyl, and Dexmedetomidine-ketamine Combinations as Sedative Agents in Pediatric Dentistry: A Triple-Blinded Randomized Controlled Trial. Contemp Clin Dent. 2018 Sep;9(Suppl 2):S197-S203. [DOI:10.4103/ccd.ccd_818_17] [PMID] []
16. Clinical Affairs Committee-Behavior Management Subcommittee, American Academy of Pediatric Dentistry. Guideline on Behavior Guidance for the Pediatric Dental Patient. Pediatr Dent. 2015 Sep-Oct;37(5):57-70.
17. Almaeen SH, Alam MK, Alruwaili HO, Alshammri BS. Comparative Study of Behavior Management Techniques in Pediatric Dentistry. J Pharm Bioallied Sci. 2024 Jul;16(Suppl 3):S2679-S2681. [DOI:10.4103/jpbs.jpbs_362_24] [PMID] []
18. Hamod MN, Kouchaji C, Rostom F, Alzoubi H, Katbeh I, Tuturov N. Evaluation of the Efficacy of Nasal Sedation Midazolam Compared with Dexmedetomidine in the Management of Uncooperative Children with Down Syndrome during Dental Treatment. Int J Dent. 2022; 2022:7344928. [DOI:10.1155/2022/7344928] [PMID] []
19. Peng L, Morford KL, Levander XA. Benzodiazepines and Related Sedatives. Med Clin North Am. 2022 Jan;106(1):113-29. [DOI:10.1016/j.mcna.2021.08.012] [PMID]
20. Hashimoto K, Zhao M, Zhu T, Wang X, Yang J. Ketamine and its two enantiomers in anesthesiology and psychiatry: A historical review and future directions. J Anesth Trans Med. 2024;3(3):65-75. [DOI:10.1016/j.jatmed.2024.07.001]
21. Attri JP, Sharan R, Makkar V, Gupta KK, Khetarpal R, Kataria AP. Conscious Sedation: Emerging Trends in Pediatric Dentistry. Anesth Essays Res. 2017 Apr-Jun;11(2):277-81. [DOI:10.4103/0259-1162.171458] [PMID] []
22. Heinz P, Geelhoed GC, Wee C, Pascoe EM. Is atropine needed with ketamine sedation? A prospective, randomised, double blind study. Emerg Med J. 2006 Mar;23(3):206-9. [DOI:10.1136/emj.2005.028969] [PMID] []
23. Chidambaran V, Costandi A, D'Mello A. Propofol: a review of its role in pediatric anesthesia and sedation. CNS Drugs. 2015 Jul;29(7):543-63. [DOI:10.1007/s40263-015-0259-6] [PMID] []
24. Albores-García D, Cruz SL. Fentanyl and other New Psychoactive Synthetic Opioids. Challenges to Prevention and Treatment. Rev Invest Clin. 2023;75(3):93-104. [DOI:10.24875/RIC.23000109] [PMID]
25. Galeotti A, Garret Bernardin A, D'Antò V, Ferrazzano GF, Gentile T, Viarani V, et al. Inhalation Conscious Sedation with Nitrous Oxide and Oxygen as Alternative to General Anesthesia in Precooperative, Fearful, and Disabled Pediatric Dental Patients: A Large Survey on 688 Working Sessions. Biomed Res Int. 2016;2016:7289310. [DOI:10.1155/2016/7289310] [PMID] []
26. Bahrami Gorji F, Amri P, Shokri J, Alereza H, Bijani A. Sedative and Analgesic Effects of Propofol-Fentanyl Versus Propofol-Ketamine During Endoscopic Retrograde Cholangiopancreatography: A Double-Blind Randomized Clinical Trial. Anesth Pain Med. 2016;6(5):e39835. [DOI:10.5812/aapm.39835] [PMID] []
27. Dal T, Sazak H, Tunç M, Sahin S, Yılmaz A. A comparison of ketamine-midazolam and ketamine-propofol combinations used for sedation in the endobronchial ultrasound-guided transbronchial needle aspiration: a prospective, single-blind, randomized study. J Thorac Dis. 2014 Jun;6(6):742-51.
28. Guan M, Wang EB, Liu Y, Zhang W. Evaluation of propofol target controlled infusion with fentanyl intravenous sedation on the removal of impacted wisdom tooth. Beijing Da Xue Xue Bao Yi Xue Ban. 2014 Feb 18;46(1):107-10.
29. Thakur S, Verma K, Singhal P, Chauhan D. Evaluation of Efficacy of Oral Ketamine and Midazolam Combination Drug in Different Doses in Different Groups Used for Moderate Sedation in Pediatric Dentistry Randomized-comparative Trial. Int J Clin Pediatr Dent. 2021;14(Suppl 2):S151-6. [DOI:10.5005/jp-journals-10005-2096] [PMID] []
30. Khatavkar SS, Bakhshi RG. Comparison of nasal Midazolam with Ketamine versus nasal Midazolam as a premedication in children. Saudi J Anaesth. 2014 Jan;8(1):17-21. [DOI:10.4103/1658-354X.125904] [PMID] []
31. das Neves JF, das Neves Araújo MM, de Paiva Araújo F, Ferreira CM, Duarte FB, Pace FH, et al. Colonoscopy sedation: clinical trial comparing propofol and fentanyl with or without midazolam. Braz J Anesthesiol. 2016 May-Jun;66(3):231-6. [DOI:10.1016/j.bjane.2014.09.014] [PMID]
32. Barkan S, Breitbart R, Brenner-Zada G, Feldon M, Assa A, Toledano M, et al. A double-blind, randomised, placebo-controlled trial of oral midazolam plus oral ketamine for sedation of children during laceration repair. Emerg Med J. 2014 Aug;31(8):649-53. [DOI:10.1136/emermed-2012-202189] [PMID]
33. Ghai B, Grandhe RP, Kumar A, Chari P. Comparative evaluation of midazolam and ketamine with midazolam alone as oral premedication. Paediatr Anaesth. 2005 Jul;15(7):554-9. [DOI:10.1111/j.1460-9592.2004.01523.x] [PMID]
34. Ahmed SS, Hicks SR, Slaven JE, Nitu ME. Deep Sedation for Pediatric Dental Procedures: Is this a Safe and Effective Option? J Clin Pediatr Dent. 2016;40(2):156-60. [DOI:10.17796/1053-4628-40.2.156] [PMID]
35. Soleimanpour H, Mahmoodpoor A, Eftekhari Milani F, Shahsavari Nia K, Mehdizadeh Esfanjani R, Safari S. Effectiveness of oral ketamine, midazolam, and atropine cocktail versus oral diphenhydramine for pediatric sedation in the emergency department. Iran Red Crescent Med J. 2014 Sep 5;16(9):e21366. [DOI:10.5812/ircmj.21366]
36. Majidinejad S, Taherian K, Esmailian M, Khazaei M, Samaie V. Oral Midazolam-Ketamine versus Midazolam alone for Procedural Sedation of Children Undergoing Computed Tomography; a Randomized Clinical Trial. Emerg (Tehran). 2015 Spring;3(2):64-9.
37. Kramer KJ, Ganzberg S, Prior S, Rashid RG. Comparison of propofol-remifentanil versus propofol-ketamine deep sedation for third molar surgery. Anesth Prog. 2012 Fall;59(3):107-17. [DOI:10.2344/12-00001.1] [PMID] []
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |


